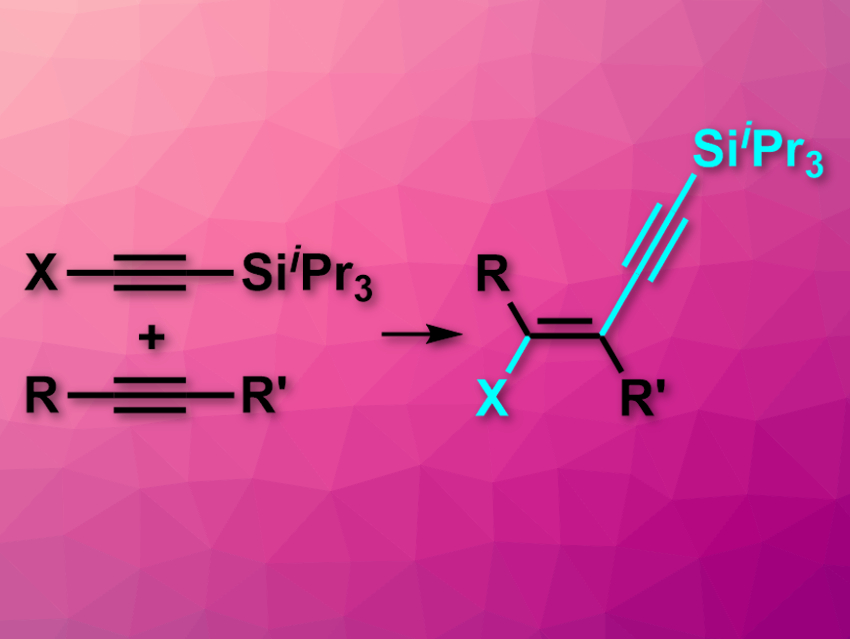
Additions of terminal alkynes across internal alkynes could be useful to synthesize 1,3-enynes, but are challenging in practice. Both the chemical selectivity and the regioselectivity need to be controlled. When it comes to stereoselectivity, existing methods are usually cis-selective. There are few examples of trans-hydroalkynylations, and no trans-chloroalkynylations had been reported so far.
Alois Fürstner, Max Planck Institute for Coal Research, Mülheim an der Ruhr, Germany, and colleagues have developed a method for efficient trans-hydroalkynylations of internal alkynes (pictured, X = H). The team also achieved the first trans-chloroalkynylation reactions using the same approach (X = Cl). They used triisopropylsilylacetylene (iPr3SiC≡CH) or (chloroethynyl)triisopropylsilane (iPr3SiC≡CCl), which were added across a variety of internal alkynes. The team used [Cp*RuCl]4 as a catalyst and 1,2-dichloroethane as the solvent to obtain the respective trans-products. The reactions were performed at 80 °C.
The desired trans-products were obtained with excellent selectivities and in generally good yields. The products are useful building blocks for further transformations.
- Ruthenium-Catalyzed trans-Hydroalkynylation and trans-Chloroalkynylation of Internal Alkynes,
Nagaraju Barsu, Markus Leutzsch, Alois Fürstner,
J. Am. Chem. Soc. 2020.
https://doi.org/10.1021/jacs.0c08582