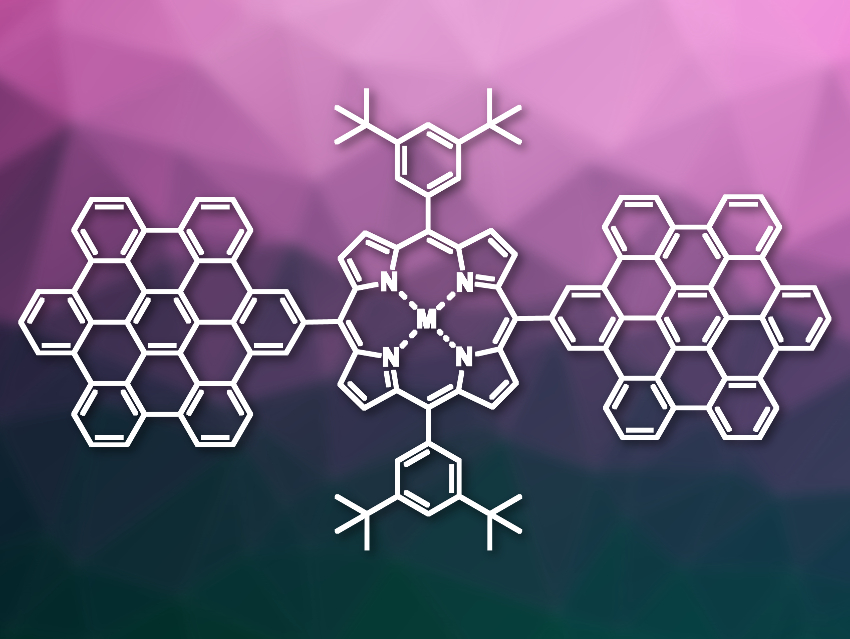
Large π-conjugated systems, such as π-extended porphyrins, can have useful optical and electronic properties. These compounds could have applications, e.g., as dyes, as organic semiconductors, or in optical materials. However, binding porphyrin to large polycyclic aromatic hydrocarbons (PAHs) can be challenging. One interesting polycyclic system is hexa-peri-hexabenzocoronene (HBC), a coronene (or superbenzene) derivative with six additional benzene rings around the edges.
Hemant K. Kashyap, Indian Institute of Technology Delhi, New Delhi, Shive M. S. Chauhan, University of Delhi, New Delhi, India, and colleagues have synthesized hexabenzocoronene–porphyrin conjugates, both in the form of the free porphyrin and as the corresponding zinc complex (pictured). The team first synthesized precursors with 4-(pentaphenylphenyl)phenyl ligands, i.e., 5,15-bis(3,5-di-tert-butylphenyl)-10,20-bis(pentaphenylphenyl)phenylporphyrin and its Zn complex, from 4-hexaphenylbenzaldehyde and 3,5-(di-tert-butylphenyl)dipyrromethane. The flexible 4-(pentaphenylphenyl)phenyl ligands were then planarized and converted to the desired hexabenzocoronenes by introducing bonds between the phenyl rings. For this, the researchers used a Scholl-type reaction with NOBF4 as an oxidizing agent.
The desired conjugates were obtained in excellent yields. Based on their electrochemical potentials, they show efficient electronic communication between the two redox-active hexabenzocoronene sites, with porphyrin acting as a π-spacer. According to the researchers, the optical properties of the conjugates indicate that they might be useful materials for light harvesting and charge transport in photovoltaic devices.
- Synthesis and Redox Properties of Superbenzene Porphyrin Conjugates,
Kharu Nisa, Vikas Khatri, Sharvan Kumar, Smriti Arora, Sohail Ahmad, Anshu Dandia, M. Thirumal, Hemant K. Kashyap, Shive M. S. Chauhan,
Inorg. Chem. 2020.
https://doi.org/10.1021/acs.inorgchem.0c01682