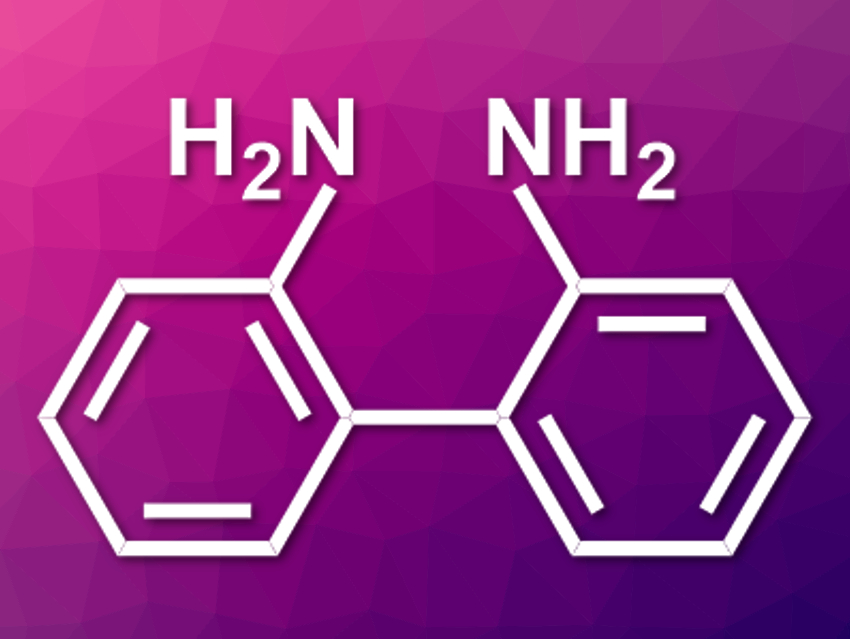
2,2′-Diaminobiaryl derivatives have applications, e.g. in catalysis, as auxiliary ligands, or in molecular imaging. These compounds can be synthesized using transition-metal-catalyzed reactions, but processes that do not require protecting groups and show high activity and selectivity are rare.
Xue-Qiang Wang, Hunan University, Changsha, China, and colleagues have developed a chemoselective, protecting-group-free, nickel-catalyzed direct homocoupling of 2-haloanilines to obtain 2,2′-diaminobiaryls (parent compound pictured). The team used 2-chloro-, 2-bromo-, or 2-iodo-functionalized aniline derivatives as substrates, NiCl2·DME (DME = dimethoxyethane) as the catalyst together with inexpensive 2,2′-bipyridine ligands, Mn powder as a reducing agent, and dimethylformamide (DMF) as the solvent. The reactions were performed at 25 °C.
The desired 2,2′-diaminobiaryls were obtained in moderate to excellent yields. The reaction has good functional group tolerance, e.g., for thioethers, silyl ethers, esters, nitriles, and heterocycles. Based on mechanistic investigations and density functional theory (DFT) calculations, the team proposes a catalytic cycle involving Ni(0), Ni(I), Ni(II), and Ni(III) species.
- Highly Chemoselective Access to 2,2′-Diaminobiaryls via Ni-Catalyzed Protecting-Group-Free Coupling of 2-Haloanilines,
Cheng-Yu Long, Shao-Fei Ni, Min-Hui Su, Xue-Qiang Wang, Weihong Tan,
ACS Catal. 2020.
https://doi.org/10.1021/acscatal.0c03428