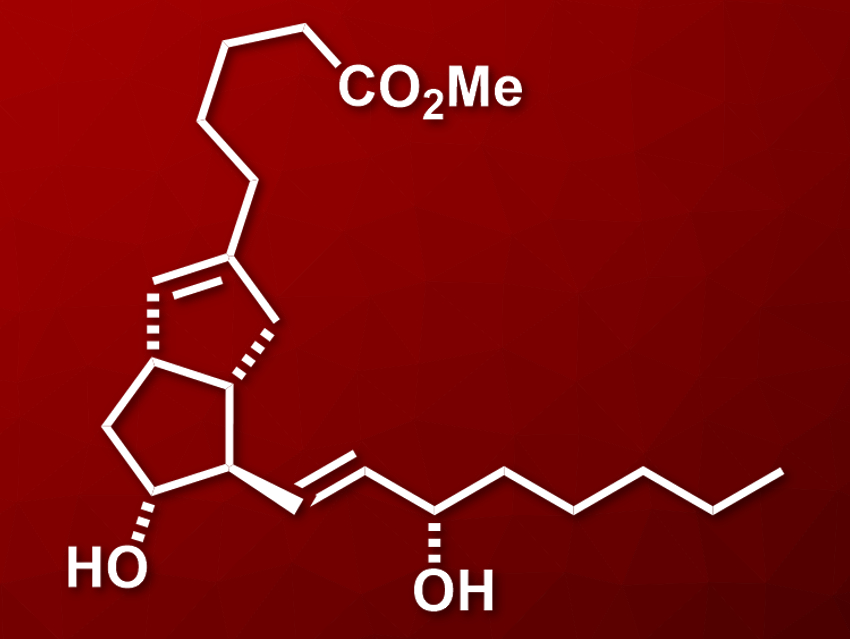
Prostacyclin, or epoprostenol, is a drug used to treat certain types of hypertension and to inhibit the formation of blood clots. However, it has a very short half-life in the body, and more stable analogues have been synthesized to improve this property. Examples of such analogues are carbacyclin and clinprost (pictured), the methyl ester of isocarbacyclin. The central chiral bicyclo[3.3.0]octene structure of clinprost and similar compounds is a challenging target for organic synthesis. An efficient process for the asymmetric synthesis of clinprost would be useful.
Nariyoshi Umekubo and Yujiro Hayashi, Tohoku University, Sendai, Japan, have developed a pot-economical, asymmetric total synthesis of clinprost. The team started from ethyl 4-oxo-2-pentenoate and 3-(triphenylsilyl)propenal and used an asymmetric domino Michael/Michael reaction catalyzed by a diphenylprolinol silyl ether to obtain the first five-membered ring in a one-pot sequence. Another multi-step one-pot reaction sequence was then used to create a ketone intermediate, which reacted in an intramolecular Horner–Wadsworth–Emmons reaction to give the bicyclo[3.3.0]octenone core. A Suzuki–Miyaura coupling reaction and a second Horner–Wadsworth–Emmons reaction were used to install the side chains.
Clinprost was obtained via seven pots in 17 % total yield and with excellent enantioselectivity. The use of several sequential one-pot transformations makes the synthesis particularly efficient, minimizes waste, and saves time.
- Pot-Economical Total Synthesis of Clinprost,
Nariyoshi Umekubo, Yujiro Hayashi,
Org. Lett. 2020.
https://doi.org/10.1021/acs.orglett.0c03616