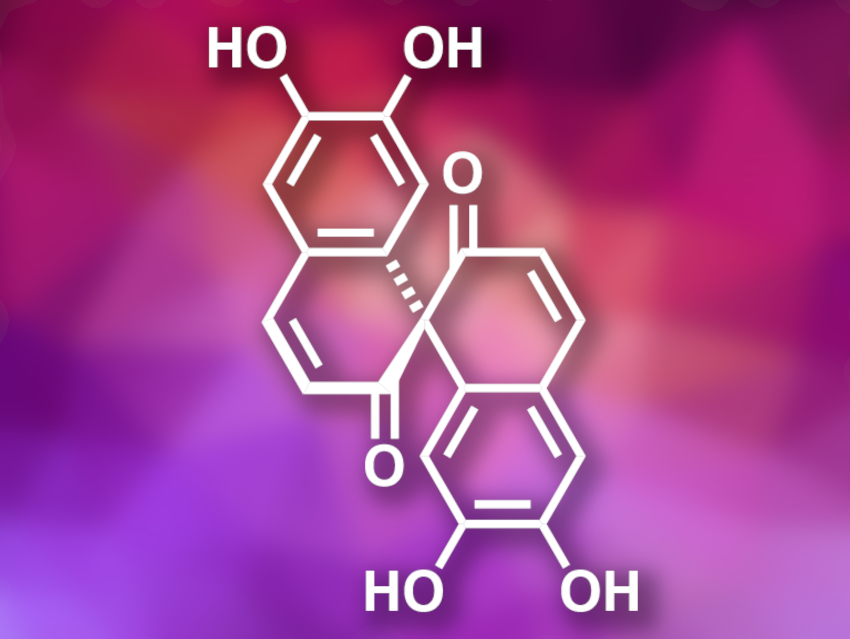
Spirobisnaphthalenes are a family of natural products, composed of two naphthalene-type units, that have been isolated from fungi and plants. Usually, the two naphthalene subunits are linked by oxygenated spiro heterocycles. Spiroaxillarone A (pictured) is a member of this family and was isolated from the plant Cyanotis axillaris. It is a symmetric spirobisnaphthalene without the typical oxygenated spiro heterocycles. Spiroaxillarone A has antimalarial activity, which makes it a potential lead compound for drug development.
Zhixiang Xie, Lanzhou University, China, and colleagues have performed the first total synthesis of (±)-spiroaxillarone A. The team started from tetrahydrocurcumin, which was protected using triisopropylsilyl (TIPS) and methyl ether groups. The protected compound was then converted to a spiro-bridged species using Mn(OAc)3 as an oxidant. The resulting intermediate was deprotected with tetra-n-butylammonium fluoride (TBAF) and BBr3. In a final one-pot transformation, the phenolic hydroxyl groups were protected using tert-butyldimethylsilyl triflate (TBSOTf) while the ketones were converted into enol silyl ethers, followed by oxidative dehydrogenation using 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) and deprotection with TBAF to give the desired product.
The researchers obtained (±)-spiroaxillarone A in five steps with an overall yield of 10 % starting from tetrahydrocurcumin. The key steps in the synthesis are a free radical cycloaddition to create the spiro-bridged ring structure and the oxidation with DDQ to introduce the double bonds. According to the researchers, the developed strategy could also be useful for the preparation of other compounds with similar spirocyclic skeletons.
- Total Synthesis of (±)-Spiroaxillarone A,
Minjian Liao, Xiangxin Li, Yajuan Zheng, Zhixiang Xie,
J. Org. Chem. 2021.
https://doi.org/10.1021/acs.joc.0c02894