Imines are usually formed via condensations of carbonyl compounds and amines under acidic conditions. However, the use of unstable aldehyde substrates and acidic reaction conditions can be problematic for large-scale applications. Therefore, efficient catalytic processes for the direct oxidation of amines or the coupling of amines and alcohols are desirable. Existing catalytic reactions can have issues such as a lack of selectivity, high reaction temperatures, long reaction times, or a need for oxidants that are not environmentally friendly.
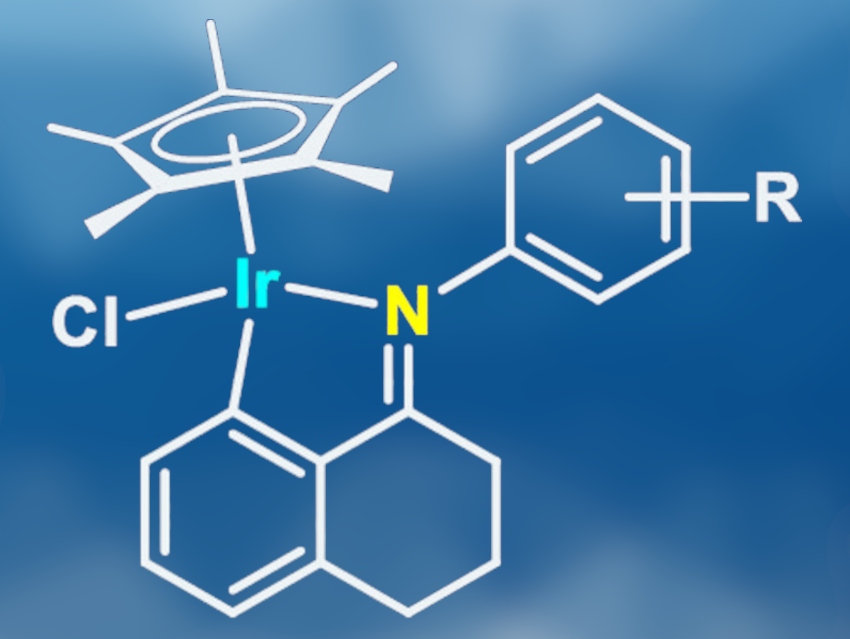
Zi-Jian Yao, Shanghai Institute of Technology, China, and Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, and colleagues have developed a series of cyclometalated iridium(III) half-sandwich complexes with Schiff base ligands (pictured, R = H, 4-Br, 4-OMe, 4-NO2) which catalyze the aerobic oxidative homocoupling of primary amines, the dehydrogenation of secondary amines, and alcohol-amine cross-coupling reactions. The ligands were synthesized from 1-tetralone and different aromatic amines in refluxing toluene. The cyclometalated Ir(III)-complexes were formed in ca. 60 % yield from the iridium precursor [Cp*IrCl2]2 and the respective ligand. The complexes are air-stable, heat-stable at 100 °C, and soluble in commonly used polar organic solvents.
Using these catalysts, the team carried out oxidative homocouplings and amine dehydrogenations with oxygen as the oxidant. The desired products were formed in excellent yields from aromatic amines as well as thiophene- or furan-based amines. Aliphatic amines showed a lower reactivity and required a higher reaction temperature and a longer reaction time. Performing the reaction in air provided sufficient oxygen for the homocoupling of primary amines, while the dehydrogenation of secondary amines required an oxygen atmosphere. The researchers were able to perform both of these reactions on a gram scale. Cross-coupling of benzylic alcohols and aromatic or benzylic amines also gave the desired products in high yields, with only small amounts of homocoupling byproducts.
- Cyclometalated Half-Sandwich Iridium(III) Complexes: Synthesis, Structure, and Diverse Catalytic Activity in Imine Synthesis Using Air as the Oxidant,
Rong-Jian Li, Chun Ling, Wen-Rui Lv, Wei Deng, Zi-Jian Yao,
Inorg. Chem. 2021.
https://doi.org/10.1021/acs.inorgchem.1c00174