Unusual structures such as planar tetracoordinate carbon atoms (ptCs) can be stabilized under certain conditions. Theoretical calculations can be used to predict if a chemical system can adopt such a rare arrangement. Various systems with planar tetracoordinate atoms with 18 valence electrons have been predicted, e.g., CAl2Si2, BAlSi3, and NAl4−. However, there had been no theoretically predicted or experimentally characterized systems with a planar tetracoordinate fluorine (ptF) atom so far.
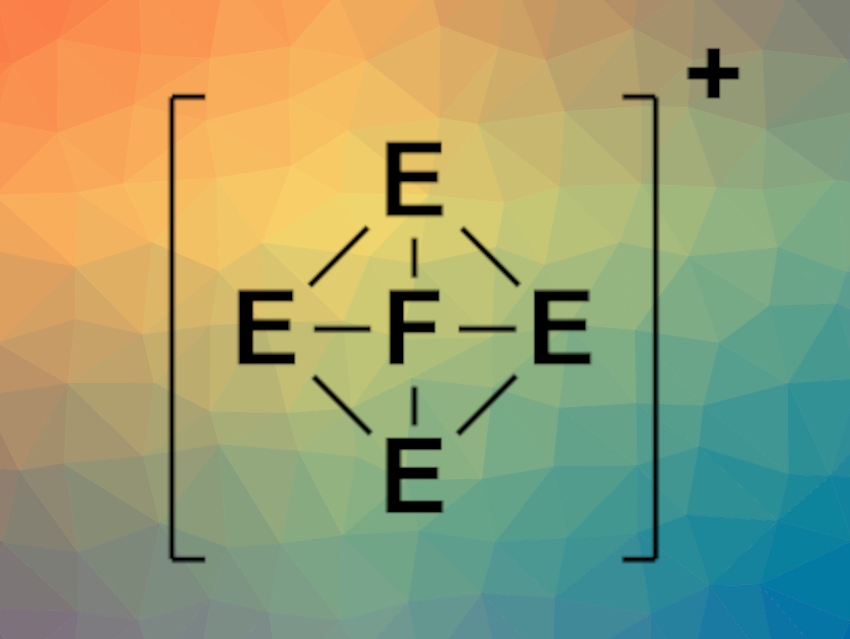
In a systematic theoretical study, Jorge Barroso, Gabriel Merino, Centro de Investigación y de Estudios Avanzados, Unidad Mérida, México, and colleagues have identified the first six clusters whose global minima on the potential energy surface (PES) contain a planar tetracoordinate fluorine, namely, FIn4+, FTl4+, FGaIn3+, FIn2Tl2+, FIn3Tl+, and FInTl3+ (general structure pictured). The team explored the PESs of 440 pentaatomic clusters, containing one fluorine atom and four main group element atoms, with 18 valence electrons and charges from +2 to −2. They used a modified genetic algorithm to search for the structures with the global energy minimum that are formed by these combinations of atoms. The bonding properties of the optimized structures were also analyzed using computational methods.
For the six compounds listed above, the researchers found global minima with a ptF atom. All of them contain the larger group 13 elements. The smaller aluminium does not form global minima structures with ptF atoms. Bonding analyses show that the interactions of fluorine with the surrounding atoms have a strongly electrostatic character and that, in contrast to planar tetracoordinate systems with, e.g., carbon, fluorine does not act as a σ-acceptor.
- Planar tetracoordinate fluorine atoms,
Gabriela Castillo-Toraya, Mesías Orozco-Ic, Eugenia Dzib, Ximena Zarate, Filiberto Ortíz-Chi, Zhong-hua Cui, Jorge Barroso, Gabriel Merino,
Chem. Sci. 2021.
https://doi.org/10.1039/d1sc01325d