Carbazole units, which consist of two benzene rings fused to the sides of a five-membered nitrogen-containing ring, can be found in dyes, but they can also act as ligands for luminescent metal complexes. They can be further functionalized at the aromatic rings to create pincer ligands with multiple binding sites—e.g., by including carbene groups. Triazole-based carbenes, for example, could be interesting for applications in luminescent complexes.
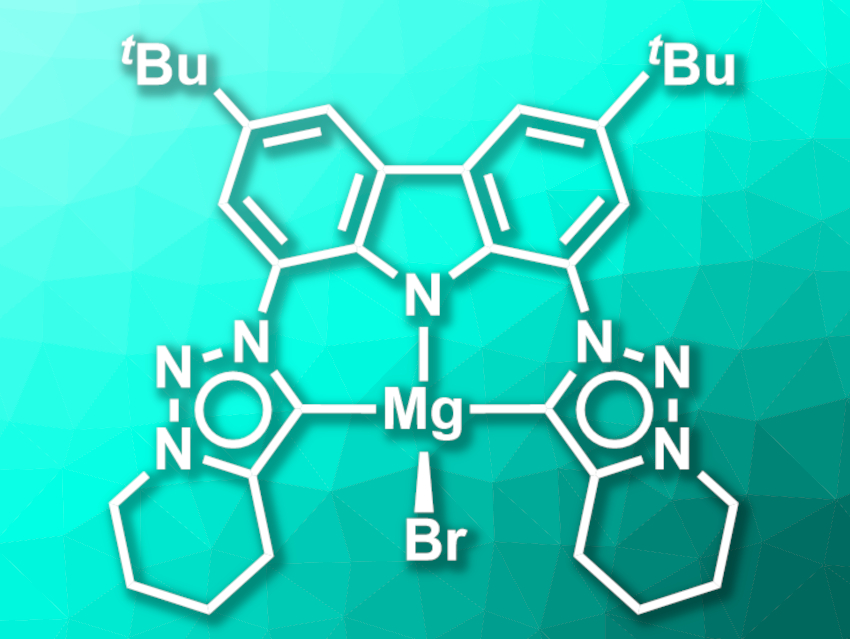
Stephan Hohloch, University of Innsbruck, Austria, Dirk M. Guldi, Friedrich-Alexander University Erlangen-Nürnberg, Erlangen, Germany, Dominik Munz, Friedrich-Alexander University Erlangen-Nürnberg and Saarland University, Saarbrücken, Germany, and colleagues have synthesized a CNC-type pincer ligand composed of a carbazole unit and two mesoionic triazole-based carbenes, which forms luminescent lithium- and magnesium complexes (latter pictured). The team first prepared a ligand precursor starting from a di-tert-butyl- and diazide-functionalized carbazole, which was reacted with 6-chlorohex-1-yne in a Cu-catalyzed azide–alkyne cycloaddition to form the triazole rings. Then, the saturated six-membered rings were closed by adding potassium iodide, resulting in an N-fused triazolium salt.
The lithium and magnesium complexes were then synthesized from the ligand precursor. Reacting the precursor with an excess of lithium hexamethyl disilazide (LiHMDS) gave a chelated, multinuclear lithium complex with a strong blue luminescence. This lithium complex can be transmetalated via a reaction with MgBr2 to give a mononuclear magnesium complex (pictured) with a lime-green luminescence. The complexes show luminescence quantum yields of up to 16 % at ambient temperature. The ligand precursor and the mono-deprotonated form of the ligand, in contrast, are essentially non-luminescent.
- Bright luminescent lithium and magnesium carbene complexes,
Piermaria Pinter, Christoph M. Schüßlbauer, Fabian A. Watt, Nicole Dickmann, Regine Herbst-Irmer, Bernd Morgenstern, Annette Grünwald, Tobias Ullrich, Michael Zimmer, Stephan Hohloch, Dirk M. Guldi, Dominik Munz,
Chem. Sci. 2021.
https://doi.org/10.1039/d1sc00846c