The emergence of new viral diseases like COVID-19 is a great challenge for public health. When a new virus begins to infect people, specific treatments and vaccinations are not available yet. Therefore, broad-spectrum antiviral drugs could be important tools for controlling the spread of such diseases.
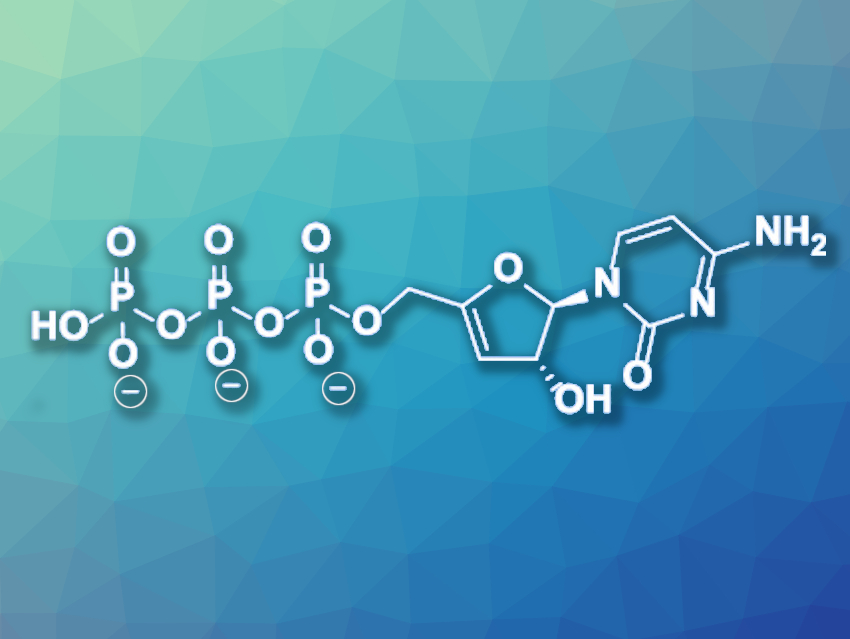
3′-Deoxy-3′,4′-didehydro-cytidine triphosphate (ddhCTP, pictured) is a naturally occurring antiviral nucleotide analogue that is produced by the enzyme viperin in vivo and has been shown to act against different viral pathogens, including SARS-CoV-2. However, the bioenzymatic synthesis using isolated viperin only gives small yields, which are not sufficient for a full evaluation of the biological activities of ddhCTP and its derivatives.
Lawrence D. Harris, Victoria University of Wellington and The University of Auckland, both New Zealand, and colleagues have developed a chemical synthesis route to ddhCTP using an eight-step reaction sequence with an overall yield of 19 %, starting from commercially available 4-N-benzoylcytidine. The selective conversion of this starting material requires the use of additional protecting groups. The team chose a tert-butyldimethylsilyl (TBDMS) group, whose lipophilic silyl groups could also simplify the chromatographic purification of the phosphorylated product. The 3′-deoxy-3′,4′-didehydroribo unit was formed by iodination at the 3′-position of the 4-N-benzoylcytidine ribose ring and a subsequent base-mediated elimination of HI.
The choice of the silyl protecting group strategy proved effective, enabling rapid reverse-phase chromatographic purification of the product after phosphorylation—instead of the challenging ion-exchange chromatography methods required for the purification of similar compounds. The removal of the protecting groups was carried out smoothly under mild conditions. The developed synthesis route provides sufficient quantities of phosphorylated ddhC derivatives, including ddhCTP, to enable further biological studies.
- Chemical Synthesis of the Antiviral Nucleotide Analogue ddhCTP,
James M. Wood, Gary B. Evans, Tyler L. Grove, Steven C. Almo, Scott A. Cameron, Richard H. Furneaux, Lawrence D. Harris,
J. Org. Chem. 2021.
https://doi.org/10.1021/acs.joc.1c00761