Amides are an important class of chemical compounds with a wide range of applications in medicine and industry. Common amide synthesis routes often require harsh reaction conditions or toxic reagents. A promising alternative to these reactions is the transition-metal-catalyzed synthesis of amides from aldehydes and hydroxylamines via an aldoxime-intermediate. Many transition-metal complexes are highly sensitive against air and moisture, which makes their use in large-scale synthesis difficult and expensive. However, half-sandwich complexes often show high stability, making them promising for use in catalysis.
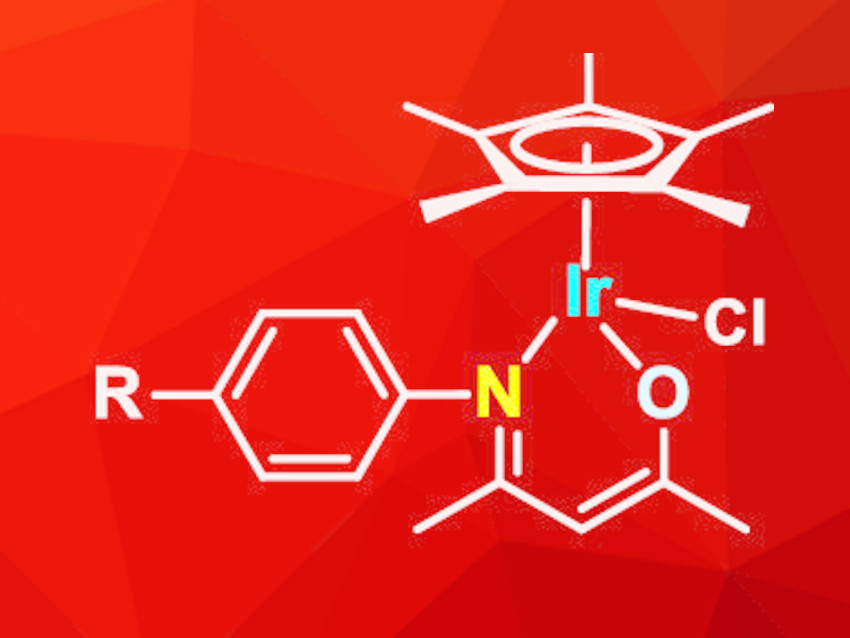
Zi-Jian Yao, Shanghai Institute of Technology, China, and Anhui Normal University, Wuhu, China, and colleagues have developed a series of iridium half-sandwich complexes with N-O-chelate-β-ketoamino ligands (pictured). The ligands were synthesized from acetylacetone and aniline derivatives in refluxing ethanol, catalyzed by acetic acid. Reactions of the ligands with [Cp*IrCl2]2 in methanol in the presence of NaHCO3 led to the formation of the desired iridium complexes in yields of 60–70 %. The complexes are stable in air for several weeks, both in solution and in the solid state.
The synthesis of benzamide from benzaldehyde and hydroxylamine hydrochloride was used as a test reaction to optimize the catalytic performance. The best results were observed at 50 °C with 0.1 mol% catalyst, NaHCO3, and 6 h reaction time in water under open-flask conditions. Under the optimized conditions, good yields were achieved with a wide range of aromatic aldehydes with electron-donating or electron-withdrawing functional groups. The reaction was also carried out successfully with heterocyclic and aliphatic aldehydes. Upscaling of the catalytic reaction to the gram scale was also effective and gave the desired products in isolated yields of over 80 %.
This new amide synthesis reaction could be a promising alternative to other procedures for industrial applications due to its efficiency, the broad substrate scope, the high stability of the catalyst, and the environmentally benign use of water as the solvent.
- Half-Sandwich Iridium Complexes Based on β-Ketoamino Ligands: Preparation, Structure, and Catalytic Activity in Amide Synthesis,
Yang Wang, Wen Guo, Ai-Lin Guan, Shuang Liu, Zi-Jian Yao,
Inorg. Chem. 2021.
https://doi.org/10.1021/acs.inorgchem.1c01530