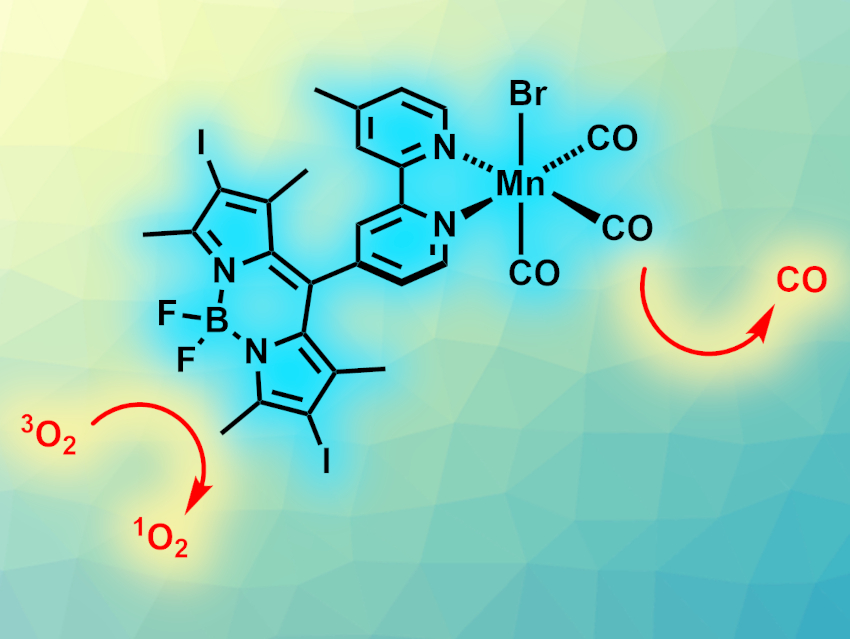
Reactive oxygen species (ROS) can interfere with cellular functions, which is useful, e.g., in cancer therapy. Carbon monoxide (CO) can increase the sensitivity of some types of cancer cells to ROS such as singlet oxygen (1O2). Photoactivated CO-releasing molecules can be used to exploit this effect by releasing CO in a targeted manner.
Shabnam Pordel, Ohio University, Athens, USA, and colleagues have developed a manganese complex with a 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY) dye unit (pictured), which combines the visible-light-activated production of 1O2 and the release of CO in one molecule. The complex, called Mn-bpy-I-BDP, was synthesized from the corresponding bipyridine ligand and Mn(CO)5Br. The bipyridine ligand was prepared starting from 4,4′-dimethyl-2,2′-bipyridine, which was converted to 4-carbaldehyde-4′-methyl-2,2′-bipyridine and then reacted with dimethylpyrrole and BF3·OEt2 to create the BODIPY unit. Finally, the iodine atoms were introduced via a reaction with N-iodosuccinimide (NIS).
The iodination of the ligand causes a red-shift in the light absorption of the complex and pushes its absorption maximum further into the visible-light region. It also increases the dark stability of the complex compared with a noniodinated derivative. In the dark, the complex is stable, but under visible LED light, it releases CO and allows the production of singlet oxygen.
- Release of CO and Production of 1O2 from a Mn-BODIPY Photoactivated CO Releasing Molecule with Visible Light,
Shabnam Pordel, Rachael N. Pickens, Jessica K. White,
Organometallics 2021.
https://doi.org/10.1021/acs.organomet.1c00331