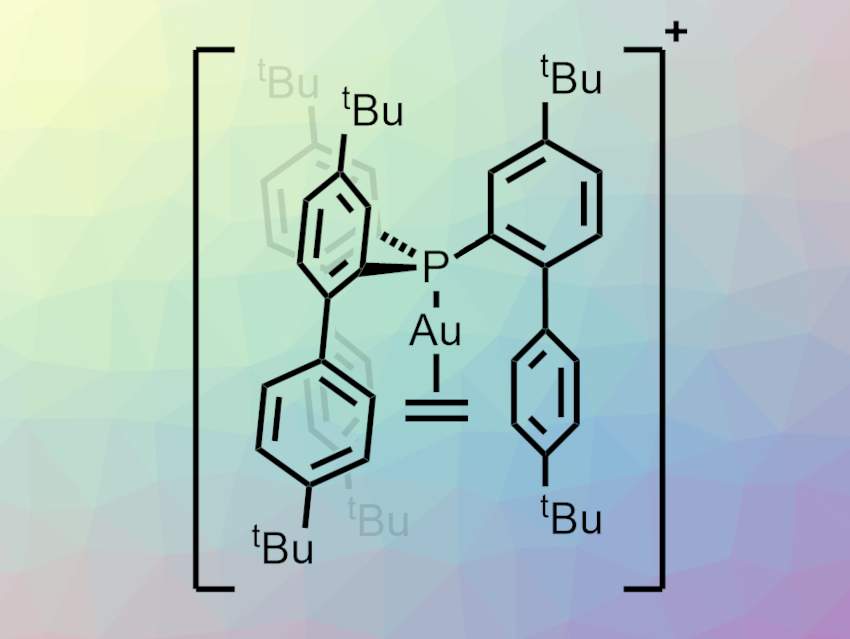
Catalysis using gold complexes can be useful in organic synthesis. Understanding key intermediates can help to optimize such processes. Gold π-complexes with unsaturated compounds, for example, have been proposed as intermediates. While there are known examples of dicoordinate cationic gold(I) complexes of substituted alkenes, no dicoordinate ethylene complexes had been isolated so far.
Israel Fernández, Universidad Complutense de Madrid, Spain, Jesús Campos, Consejo Superior de Investigaciones Científicas (CSIC) and University of Sevilla, Sevilla, Spain, and colleagues have synthesized the first dicoordinate gold(I)–ethylene complex (pictured). The team used a bulky tris-2-(4,4′-di-tert-butylbiphenylyl)phosphine ligand to stabilize this unusual complex. They first reacted the phosphine ligand with [AuCl(THT)] (THT = tetrahydrothiophene) to form a neutral phosphine chloride complex. This intermediate was reacted with AgSbF6 under an ethylene atmosphere, forming an AgCl precipitate and the desired cationic Au(I) ethylene complex.
According to the researchers, the bulky ligand forms a cavity that kinetically stabilizes the ethylene complex. Bonding analysis was performed using density functional theory (DFT) calculations. The team found that, in contrast to related tricoordinate gold(I)–ethylene complexes, π-backdonation plays a minor role in the bond between gold and ethylene.
- A dicoordinate gold(I)–ethylene complex,
Miquel Navarro, Juan Miranda-Pizarro, Juan J. Moreno, Carlos Navarro-Gilabert, Israel Fernández, Jesús Campos,
Chem. Commun. 2021.
https://doi.org/10.1039/d1cc02769g