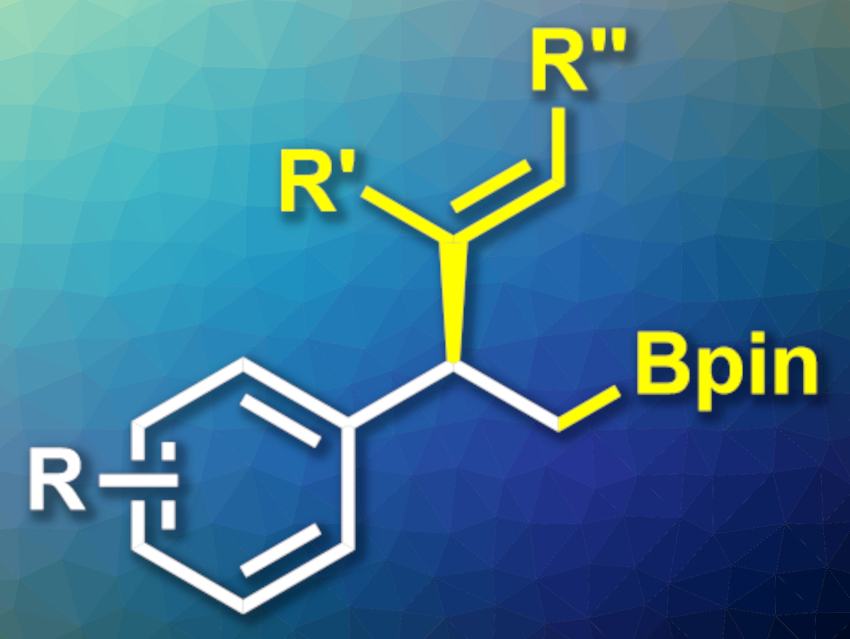
The difunctionalization of alkenes is a useful type of reaction in organic synthesis. Carboborations, for example, can lead to suitable intermediates for further transformations. Vinylborations, in particular, introduce two functional groups at the same time. Enantioselective versions of this reaction can, thus, be useful.
Yang Ye, Tian Xie, Xiang-Yang Ye, Hangzhou Normal University, Zhejiang, China, and colleagues have developed a nickel-catalyzed asymmetric 1,2-vinylboration reaction of styrene derivatives that uses a chiral bisoxazoline ligand (example product pictured). The team used a complex of NIBr2 and a cyclopropanyl-functionalized bisoxazoline ligand of the type 2,2’-(cyclopropane-1,1-diyl)bis(3a,8a-dihydro-8H-indeno[1,2-d]-oxazole as the catalyst, different styrene derivatives as the substrates, a variety of alkenyl bromides as the reactants, bis(pinacolato)diboron as the boron source, LiOMe as a base, and 1,4-dioxane as the solvent. The reactions were performed at 10 °C.
The desired products were obtained in moderate to good yields and with mostly excellent enantioselectivities. The protocol tolerates a variety of functional groups and could be useful, e.g., in the synthesis of natural products and pharmaceutically active compounds.
- Nickel-Catalyzed Enantioselective 1,2-Vinylboration of Styrenes,
Yang Ye, Jiandong Liu, Bing Xu, Songwei Jiang, Renren Bai, Shijun Li, Tian Xie, Xiang-Yang Ye,
Chem. Sci. 2021.
https://doi.org/10.1039/d1sc04071e