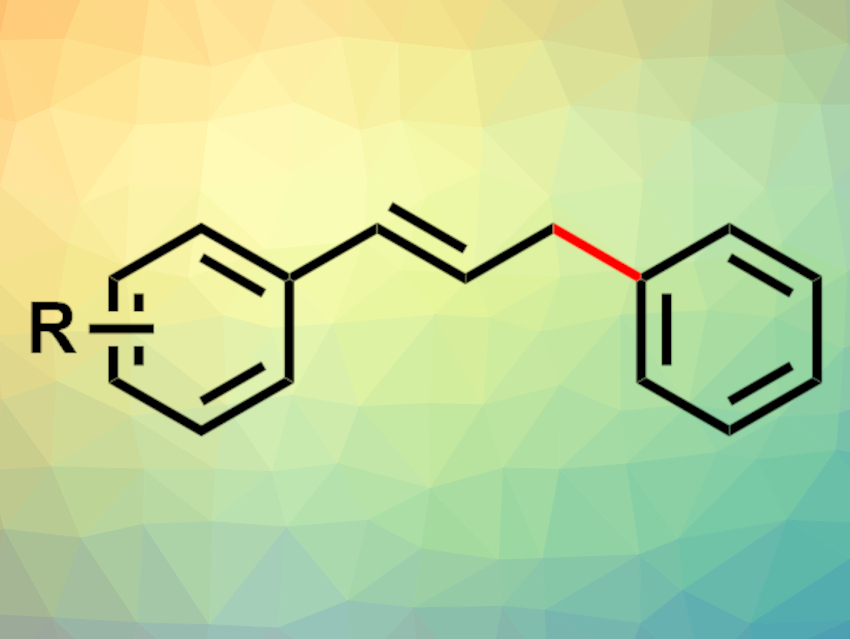
Transition-metal-catalyzed cross-coupling reactions are important tools in organic synthesis. Often, palladium-based catalysts are used, but earth-abundant metals such as nickel could be useful alternatives for many substrates.
Huifei Wang, Ningbo University, Peking University Shenzhen Graduate School, Nanjing University, all China, Hongze Liang, Ningbo University, and colleagues have developed a nickel-catalyzed reductive cross-coupling reaction between phosphonium salts and allylic alcohols or their derivatives (example product pictured). The team used NiBr2·Et2O as a catalyst together with 4,4′-dimethoxy-2,2′-bipyridine as a ligand, a combination of ZrCl4 and LaCl3 as a Lewis-acid additive, Mn as a stoichiometric reductant, and N,N-dimethylacetamide (DMA) as the solvent.
The desired coupling products were obtained in generally good yields. The researchers expanded the protocol to heterocyclic thiazolyl-phosphonium salts, which gave functionalized benzothiazoles in moderate to good yields. They also used the reaction on a gram scale as well as in a late-stage functionalization to demonstrate its utility.
- Nickel-Catalyzed Reductive Csp2–Csp3 Cross Coupling Using Phosphonium Salts,
Huifei Wang, Mengwan Yang, Yuting Wang, Xi Man, Xinyao Lu, Zehuai Mou, Yunjie Luo, Hongze Liang,
Org. Lett. 2021.
https://doi.org/10.1021/acs.orglett.1c02893