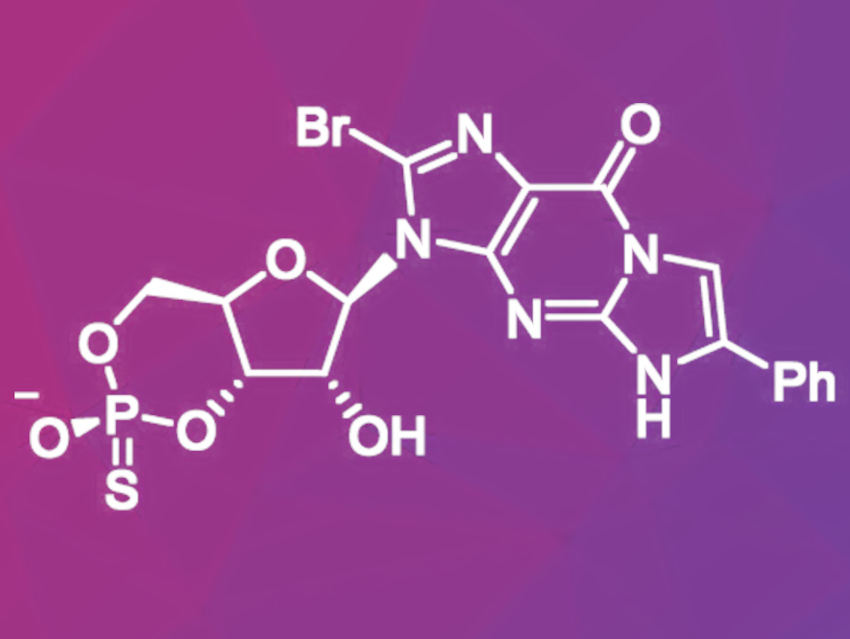
The overactivation of a class of enzymes called cGMP-dependent protein kinases (PKG) is considered to be one of the main causes of inherited retinal neurodegeneration—a disease that can lead to vision loss. This overactivation is caused by higher-than-normal amounts of cyclic guanosine monophosphate (cGMP) in photoreceptors in the retina. Therefore, PKG inhibition with cGMP analogues is a promising approach for the treatment of this disease.
Screenings of various cGMP analogues led to the discovery of a cGMP analogue with promising neuroprotective effects in laboratory studies. This molecule (pictured) is a thiophosphate-containing derivative of cGMP. Thiophosphates are more resistant against enzymatic cleavage and, therefore, more stable inside the cell. During the synthesis of this compound, high purity is important because small changes in the molecular structure could lead to compounds that increase PKG activity like natural cGMP.
Oswaldo Pérez, Research Institutes of Sweden—Chemical Processes and Pharmaceutical Development, Södertälje, and University of Iceland, Reykjavík, and colleagues have developed a preparative synthesis route for this drug candidate via an intermediate 5′-H-phosphonate ester which gave the target product in 13.8 % overall yield and over 99 % purity in six steps. First, a phenylethenyl group was introduced to 8-bromoguanosine by reacting it with 2-bromoacetophenone in the presence of the base 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU). After a selective silyl protection, the resulting intermediate was reacted with diphenyl phosphite to give the corresponding H-phophonate monoester after hydrolysis of the phenyl group. In the next step, the H-phosphonate was cyclized using pivaloyl chloride in the presence of lutidine and converted to the thiophosphate by addition of elemental sulfur and triethylamine. Finally, the protective groups were removed.
The product was successfully purified by crystallization, avoiding chromatographic purification. The final yield was 125 g, which makes this synthesis route useful for further studies on the treatment of retinal degeneration using this drug candidate.
- Preparative Synthesis of an RP-Guanosine-3′,5′-Cyclic Phosphorothioate Analogue, a Drug Candidate for the Treatment of Retinal Degenerations,
Oswaldo Pérez, Nicolaas Schipper, Martin Bollmark,
Org. Process Res. Dev. 2021.
https://doi.org/10.1021/acs.oprd.1c00230