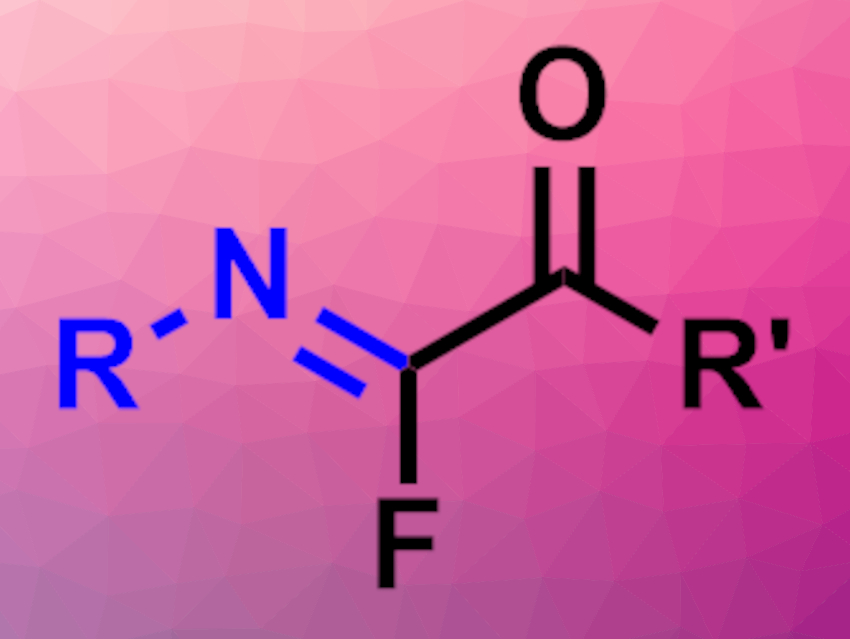
Imidoyl fluorides, i.e., compounds of the type R–C(=NR’)–F, could serve as useful intermediates in organic synthesis. However, there is a lack of general synthetic methods to access these compounds. Existing methods are hampered, e.g., by a need for harsh conditions or low regioselectivity.
Ha Eun Kim, Jun-Ho Choi, and Won-jin Chung, Gwangju Institute of Science and Technology, Republic of Korea, have synthesized α-ketoimidoyl fluorides (pictured) from geminal chlorofluorides via a tandem azidation/rearrangement. The team reacted a range of geminal chlorofluorides of the type RFClC–C(=O)-R’ with the azide nucleophile n-Bu4NN3 using 1,2-dichloroethane as the solvent. The reactions were performed at 80 °C.
The desired α-ketoimidoyl fluorides were obtained in good to excellent yields under mild conditions. The reaction proceeds via the displacement of the chlorine substituent by the azide, followed by a rearrangement and a loss of N2 to form the imidoyl fluoride. The reaction does not proceed in the absence of the fluoride substituent, which indicates that it promotes the transformation. According to the researchers, the work could allow the use of α-ketoimidoyl fluorides in the synthesis of nitrogen- and/or fluorine-containing organic compounds.
- Synthesis of α-Ketoimidoyl Fluorides via Geminal Fluorine-Promoted Azide Rearrangement,
Ha Eun Kim, Jun-Ho Choi, Won-jin Chung,
Org. Lett. 2021.
https://doi.org/10.1021/acs.orglett.1c03309