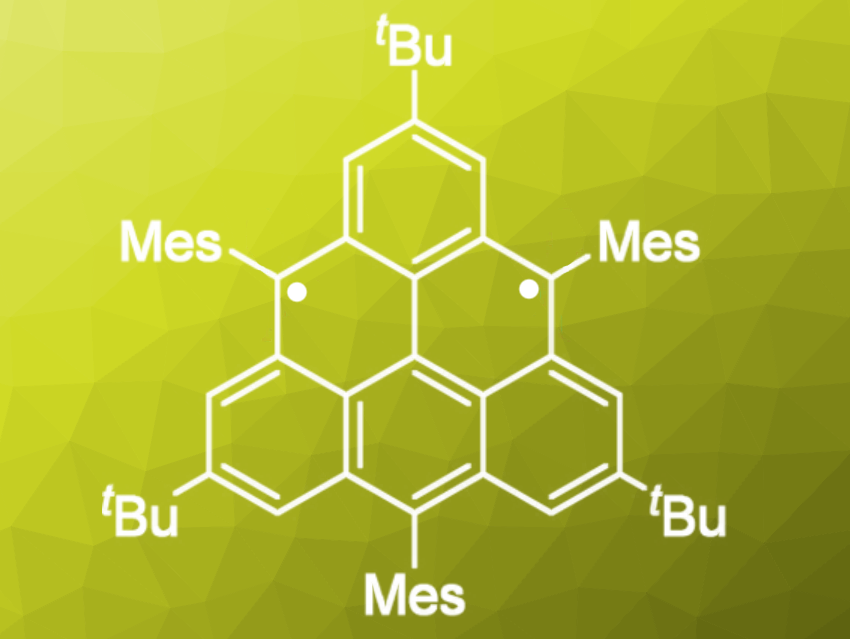
Triangulene is one of the best-known benzenoid hydrocarbons in the triplet ground state. Its unique electronic and highly symmetrical structure has led many scientists to synthesize and isolate triangulene and its derivatives. So far, however, all attempts to isolate them as crystals have been unsuccessful.
Akihiro Shimizu, Ryo Shintani, and Shinobu Arikawa, Osaka University, Japan, Daisuke Shiomi and Kazunobu Sato, Osaka City University, Japan, have synthesized and isolated a kinetically stabilized crystalline triangulene. The team added bulky substituents onto the reactive zigzag edges to prevent polymerization. Their triangulene derivative is stable at room temperature but must be kept in an inert atmosphere; it slowly degrades when exposed to oxygen.
The highly symmetrical structure of the triangulene was confirmed by X-ray crystallography, and the magnetic properties of the crystalline nanographene were studied in detail. This highly symmetric non-Kekulé molecule was theoretically estimated as a triplet diradical having a large singlet–triplet energy gap (ΔEST), and it is also a model to understand the edge state of zigzag-edged nanographene.
The scientists believe that their research opens the door to the synthesis and isolation of other hydrocarbons with higher spin multiplicity, and that it will help elucidate the edge state of nanographenes with zigzag edges with higher-spin ground states.
- Synthesis and Isolation of a Kinetically Stabilized Crystalline Triangulene,
Shinobu Arikawa, Akihiro Shimizu, Daisuke Shiomi, Kazunobu Sato, Ryo Shintani,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.1c10151
Update (November 17, 2021)
The structure originally omitted the radical centers at the Met substituents