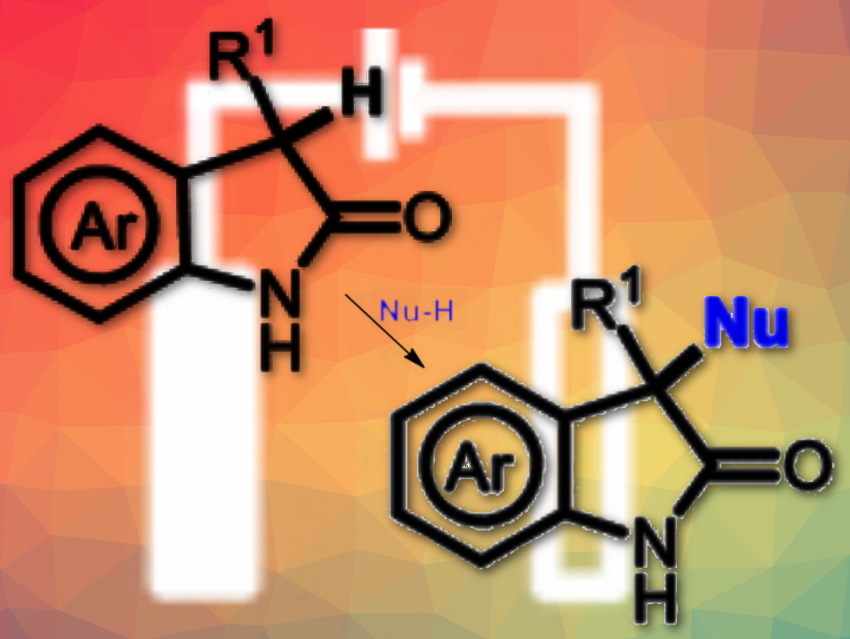
Nuno Maulide, University of Vienna, Austria, and colleagues have developed a general electrochemical method to obtain unsymmetrical 3,3-disubstituted oxindoles by direct C–H functionalization. The team used a simple undivided cell with a graphite anode and cathode, constant current of 10 mA, tetraethylammonium p-toluenesulfonate (Et4NOTs) as the electrolyte, and a mixture of MeCN/EtOH as solvent and nucleophile.
The approach allows functionalization of unsymmetrical 3,3-disubstituted oxindoles through C–O formation. It can be extended to the formation of C–C bonds and C–N bonds by using silyl enol ether and TMSN3 as nucleophiles. The approach tolerates various substituents at the C-3 position of the oxindole core (pictured above; R1) and alkyl- and aryl-substituted nitrogen atoms. The electrosynthesis allows for environmentally friendly and mild reaction conditions. The approach does not rely on stoichiometric oxidants.
The team proposes two mechanistic scenarios, depending on the nucleophilic source used and its oxidation potential:
- The substrates undergo two-electron oxidation at the anode. This results in the formation of the corresponding carbocation. This could explain the need for excess amounts of the nucleophile (in some cases used as a co-solvent), justified due to its role of acting as a proton source for hydrogen evolution at the cathode.
- A one-electron oxidation at the anode leads to the formation of a radical cation. Through proton loss, a captodative radical intermediate is generated.
- Electrochemical Umpolung C–H Functionalization of Oxindoles,
Miryam Pastor, Marie Vayer, Harald Weinstabl, Nuno Maulide,
J. Org. Chem. 2021.
https://doi.org/10.1021/acs.joc.1c02616