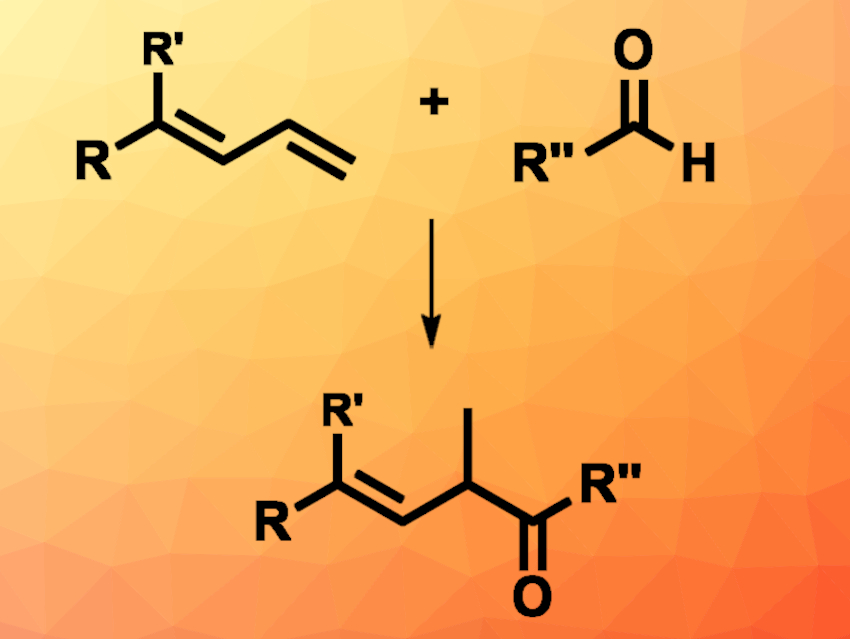
Cooperative catalysis using NHCs (N-heterocyclic carbenes) and transition metals can be a useful tool to synthesize high-value functionalized alkenes. Due to its abundance and the ability to take part in oxidative additions, nickel is used often in organometallic catalysis, for example, in the hydrofunctionalization of 1,3-dienes with different nucleophiles.
Song Ye, Beijing National Laboratory for Molecular Sciences, Chinese Academy of Sciences, and University of the Chinese Academy of Sciences, Beijing, Chun-Lin Zhang, Beijing National Laboratory for Molecular Sciences, and colleagues have used a cooperative catalysis approach employing NHCs and nickel in a hydroacylation reaction (pictured). 1,3-dienes were reacted with aliphatic or aromatic aldehydes, forming β,γ-unsaturated ketones. The team used Ni(COD)2 as the transition-metal catalyst together with the bidentate phosphine ligand Xantphos (9,9-dimethyl-9H-xanthene-4,5-diyl)bis(diphenylphosphane), a thiazolium-based NHC precursor, potassium carbonate as a base, and water as the solvent. The reactions were performed at 100 °C under an inert atmosphere.
The desired products were obtained in moderate to high yields. Some of the products were converted to the corresponding saturated ketones by a subsequent hydrogenation. Based on deuterium labeling experiments, the research team proposes a mechanism that involves a Ni-π-allyl intermediate formed from the diene and a Breslow intermediate formed from the aldehyde under NHC catalysis. According to the researchers, the protocol is the first to show the compatibility of NHC catalysis with nickel catalysis, and other NHC/nickel-catalyzed reactions might be possible.
- Cooperative N-Heterocyclic Carbene/Nickel-Catalyzed Hydroacylation of 1,3-Dienes with Aldehydes in Water,
Hao Liu, You-Feng Han, Zhong-Hua Gao, Chun-Lin Zhang, Congyang Wang, Song Ye,
ACS Catal. 2022.
https://doi.org/10.1021/acscatal.1c05517