N-fluorobenzenesulfonimide (NFSI) is widely used as a fluorinating agent. It can also be used to introduce benzenesulfonimide groups in a variety of substrates, forming new C–N bonds. The scope of this reaction is rather limited, however, which is presumably due to the steric demands of the benzenesulfonimide group. Additionally, further derivatization of the benzenesulfonimide group in the products is difficult, which diminishes the usefulness of this procedure.
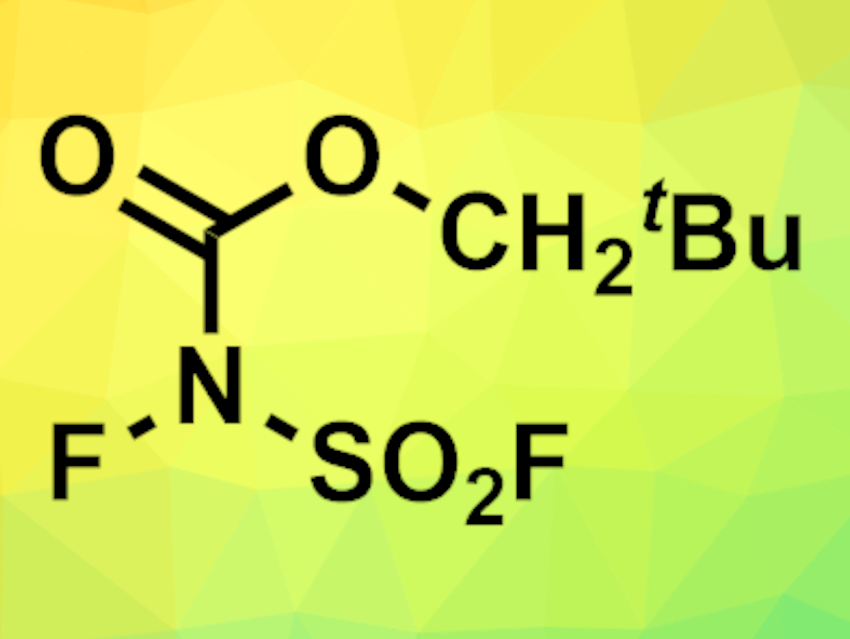
Kohsuke Aikawa, The University of Tokyo, Japan, Takuya Hashimoto, Chiba University, Japan, and colleagues have developed a new N-fluorinated imide reagent, N-fluoro-N-(fluorosulfonyl)carbamate (NFC, pictured). NFC was synthesized by treatment of the corresponding lithium amide with fluorine gas in an acetonitrile/water mixture at 0 °C. The product was readily formed in good yields without additional purification steps and is bench-stable.
The team used NFC in the copper-catalyzed C–H-imidation of heteroarenes and polyaromatic hydrocarbons. Moderate to good yields were achieved with a wide range of substrates. Benzene did not react under the initial conditions, but irradiation with blue LED light in dichloromethane led to the desired product in good yields. Mesitylene, which underwent C-H-imidiation in the benzylic position under thermal conditions, also reacted at the benzene core under irradiation. Due to this difference in reactivity under thermal or photochemical conditions, the selective formation of core-functionalized or side-chain-functionalized products from alkylated benzene derivatives is possible.
Additionally, NFC was used in the imidocyanation of aliphatic alkenes together with TMS–CN, which had not been described for NFSI previously. This reaction also gave the desired products in good yields for a wide variety of substrates. Derivatization of the products (a drawback for procedures involving NFSI) was also tested, and the introduced imide groups could be successfully transformed into free amines, sulfonamides, and sulfamides. The new process, thus, successfully overcomes two of the main issues of previous NFSI-based pathways: The small substrate scope and the difficult derivatization.
- An N-Fluorinated Imide for Practical Catalytic Imidations,
Yuno Oe, Ryuhei Yoshida, Airi Tanaka, Akiya Adachi, Yuichiro Ishibashi, Takashi Okazoe, Kohsuke Aikawa, Takuya Hashimoto,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.1c13569