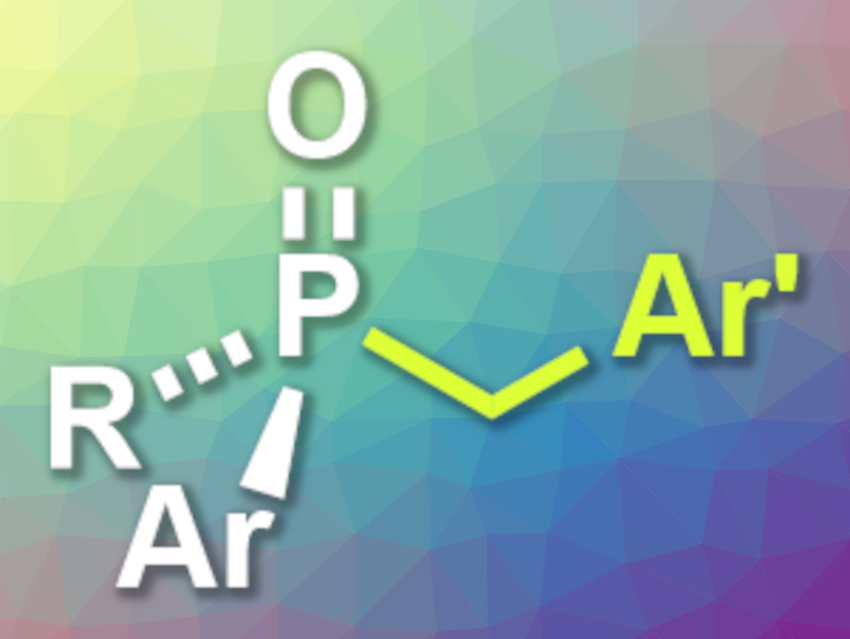
Chiral phosphine compounds with a stereocenter at the phosphorus atom have possible applications, e.g., in catalysis or medicinal chemistry. Transition-metal-catalyzed coupling reactions of phosphorus nucleophiles with electrophiles can be used to synthesize such P-chiral compounds. Secondary phosphine oxides can be useful coupling partners due to their stability and ease of handling.
Qing-Wei Zhang, University of Science and Technology of China, Hefei, and colleagues have developed a protocol for the nickel-catalyzed benzylic substitution of secondary phosphine oxides to give P-stereogenic tertiary phosphine oxides (pictured). The team used commercially available benzyl chlorides and bench-stable secondary phosphine oxides as coupling partners, Ni(cod)2 (cod = 1,5-cyclooctadiene) as the catalyst together with (S,S)-Me-Duphos as a chiral ligand, K2HPO4 as a base, iPrOH as an additive, and mesitylene as the solvent.
The desired products were obtained in moderate to high yields and with high to excellent enantioselectivities. The reaction showed good functional group tolerance. The products might be useful for further transformations to give chiral ligands or organocatalysts.
- Ni-Catalyzed Enantioselective Benzylation of Secondary Phosphine Oxide,
Wen-Qiang Cai, Qi Wei, Qing-Wei Zhang,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c00209