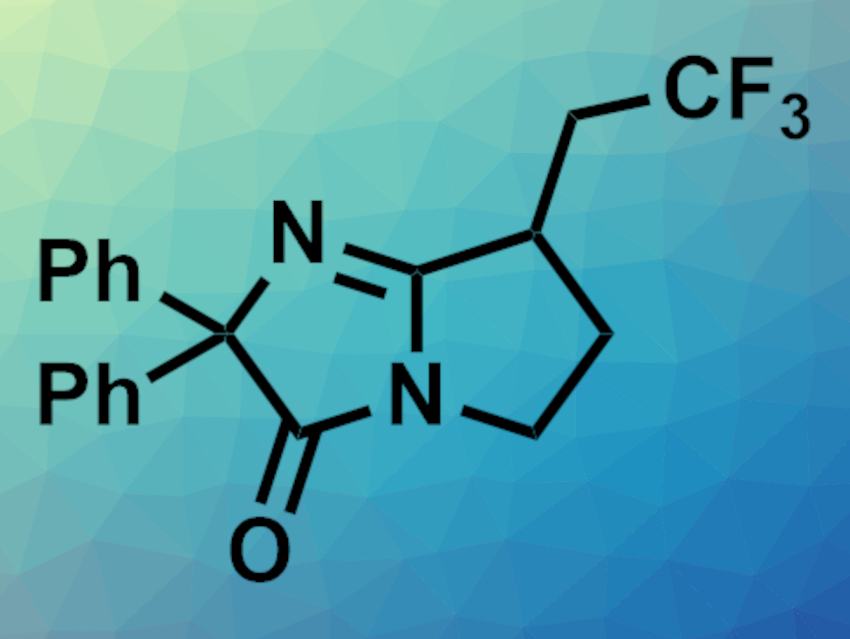
Ring-containing compounds are of interest in organic synthesis as they occur in many natural products. Transition-metal catalysis and photoredox reactions can be used to synthesize valuable cyclic compounds. However, this often generates waste, requires multiple steps, and provides limited options for functionalization.
Wei-Wei Liao, Jilin University, Changchun, China, and Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, and colleagues have developed a copper-catalyzed process in which an alkene trifluoromethylation triggers a nitrile insertion/remote functionalization relay process to give functionalized azaheterocycles (example pictured). N-cyanamide alkenes were used as reactants. The team used Cu(OAc)2 as a catalyst and 1-(trifluormethyl)-1,2-benziodoxol-3(1H)-on (Togni II reagent) as a trifluoromethylating agent in dichloromethane (DCM) at 80 °C. Using this approach, the researchers synthesized a variety of trifluoromethylated azaheterocycles in moderate to high yields.
The relay processes in the developed reactions are mediated by radical 1,n-hydrogen atom transfer, or 1,n-HAT (n = 5,6). The team proposes a mechanism that involves the formation of a trifluoromethyl radical via single-electron transfer (SET) between the Togni II reagent and the copper(I) catalyst. This radical attacks the alkene unit to form a carbon radical, followed by an intramolecular addition to the cyano group of the substrate. This gives an iminyl radical that takes part in a HAT reaction, leading to cyclization and the formation of the desired product.
- Radical Alkene-Trifluoromethylation-Triggered Nitrile Insertion/Remote Functionalization Relay Processes: Diverse Synthesis of Trifluoromethylated Azaheterocycles Enabled by Copper Catalysis,
Ji-Ming Xi, Yun-Hai Sun, Wen-Cheng Li, Yu-Heng Wu, Zhong-Lin Wei, Wei-Wei Liao,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c00083