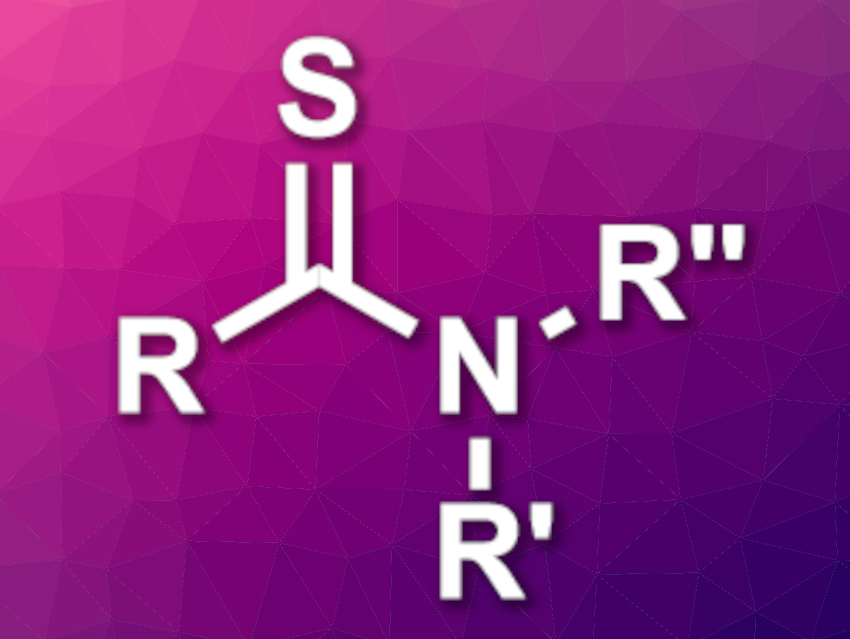
Thioamides (pictured) are useful intermediates, e.g., in the synthesis of heterocyclic compounds. They also have applications in pharmaceutical chemistry. Protocols for the synthesis of thioamides usually require oxidants, additives, catalysts, and/or hazardous reagents.
Guddeangadi N. Gururaja, Central University of Gujarat, Gandhinagar, India, and colleagues have developed an environmentally benign water-mediated thioamidation reaction at room temperature with no need for catalysts, additives, or oxidants. The team reacted different aromatic or aliphatic aldehydes with elemental sulfur and various primary or secondary amines, using water as a “green” solvent.
The desired thioamides were obtained in moderate to excellent yields. The presence of water was shown to be essential for a successful conversion. The researchers propose that a polysulfide forms from the elemental sulfur and the amine in water and then acts as a nucleophilic sulfur source for the formation of the thioamide.
- Water Mediated Direct Thioamidation of Aldehydes at Room Temperature,
Ankush Gupta, Jigarkumar K. Vankar, Jaydeepbhai P. Jadav, Guddeangadi N. Gururaja,
J. Org. Chem. 2022.
https://doi.org/10.1021/acs.joc.1c02307


![A Path to Substituted Bicyclo[2.1.1]hexanones](https://www.chemistryviews.org/wp-content/uploads/2024/10/1substitutedbicyclo211hexan2ones_2024-125x94.png)
