Metal–organic frameworks (MOFs) are porous materials composed of metal centers and organic linkers. They have applications, e.g., in catalysis or gas separation and adsorption. The coordination number of the metal centers can influence the properties of the MOF. High coordination numbers can be achieved by combining large metal centers, e.g., actinides, with small ligands. The highest coordination number in a MOF had been ten so far and was achieved using tetravalent actinides as the metal centers.
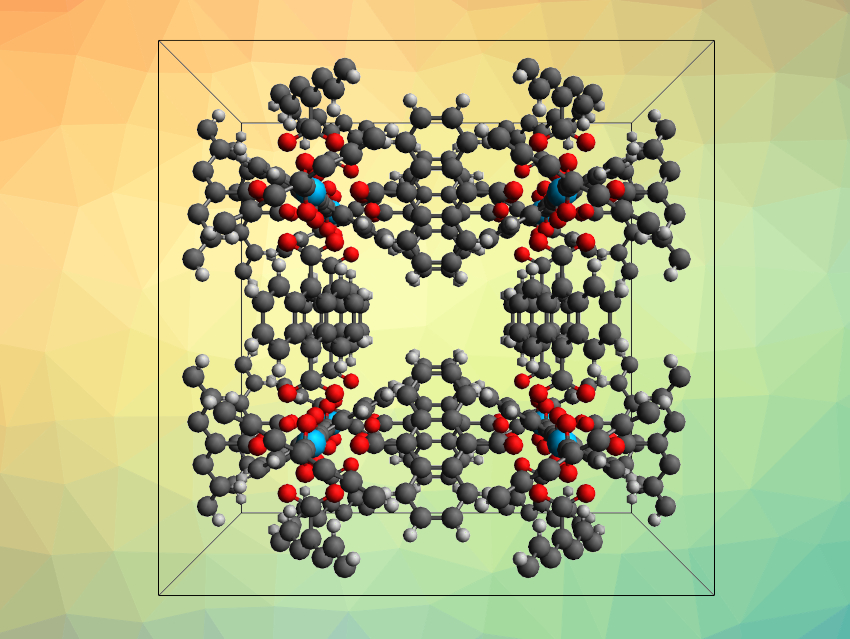
Kai Lv, China Academy of Engineering Physics (CAEP), Mianyang, China, and Helmholtz-Zentrum Dresden-Rossendorf (HZDR), Dresden, Germany, Moritz Schmidt, Helmholtz-Zentrum Dresden-Rossendorf, and colleagues have synthesized new Th-, U-, Np-, and Pu-based MOFs that raise the coordination number limit to twelve (example pictured). The team prepared MOFs of the type An(IV)-ADC (An = actinide, ADC = 9,10-anthracenedicarboxylic acid) from Th(NO3)4·5H2O, UCl4, NpCl4·2DME, or PuCl4·2DME (DME = dimethoxyethane) and the ADC ligand precursor.
The team found that in the resulting MOFs, each actinide center is coordinated by six bidentate carboxylate groups from deprotonated ADC, forming icosahedral [AnO12] units. Each ligand links two metal centers, leading to a highly symmetrical structure. The MOFs show autoluminescence due to their radioactivity. According to the researchers, the MOFs might be useful, e.g., for hard radiation detection.
- MOFs with 12-Coordinate 5f-Block Metal Centers,
Kai Lv, Christian Urbank, Michael Patzschke, Juliane März, Peter Kaden, Stephan Weiss, Moritz Schmidt,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.1c13127




Hi there. I am a researcher in the synthesis of MOFs. Can you help me with this MOF synthesis.