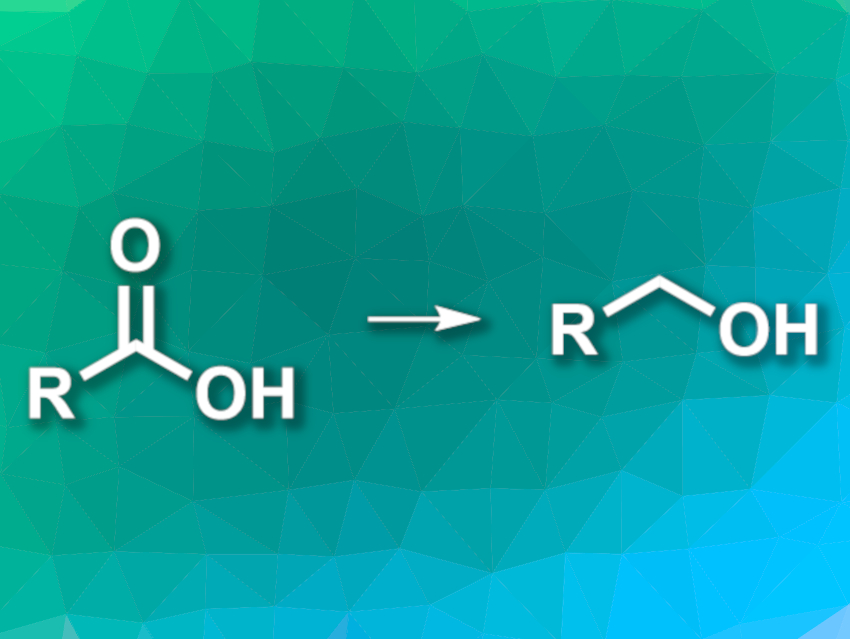
The reduction of carboxylic acids to alcohols is a useful transformation in organic synthesis. Often, stoichiometric aluminium hydride reagents are used for this type of reaction. However, these reagents can be hard to handle due to their air- and moisture sensitivity. Activating the carboxylic acids can allow reductions with milder reagents, but worsens the atom economy of the reaction due to the use of stoichiometric activating agents.
Ross M. Denton, University of Nottingham, GlaxoSmithKline Carbon Neutral Laboratories for Sustainable Chemistry, UK, and colleagues have developed a method for the reduction of carboxylic acids in which both the acid and a silane-based reductant are activated via the in–situ formation of silyl esters, allowing for a practical reduction without a need to strictly exclude air and moisture. The team used phenylsilane as a reductant, N-methylmorpholine and Zn(OAc)2 as catalysts, and 2-methyltetrahydrofuran (2-MeTHF) as the solvent. The reactions were performed at 80 °C.
Using this approach, the team was able to reduce a variety of benzoic acids, other aromatic acids, and aliphatic carboxylic acids, obtaining the corresponding alcohols in moderate to good yields. The reaction tolerates a variety of functional groups, hindered substrates such as adamantane carboxylic acid, and different protecting groups. A reduction on the gram scale was also performed. The team proposes a mechanism that involves the formation of silyl ester intermediates. According to the researchers, this concept of activating both a substrate and a silane by the formation of silyl esters could also be applied to the design of other reactions.
- In situ silane activation enables catalytic reduction of carboxylic acids,
Emma L. Stoll, Thomas Barber, David J. Hirst, Ross M. Denton,
Chem. Commun. 2022.
https://doi.org/10.1039/d1cc03396d