Catalytic hydrogenations are important reactions in chemical synthesis. Catalysts used for this type of reaction usually contain noble metals like platinum or palladium. These metals are highly active but expensive. Therefore, the substitution of noble metal catalysts by more abundant elements is desirable. Iron is Earth-abundant and has low inherent toxicity. However, unlike platinum or palladium, iron needs suitable ligands for use in catalytic hydrogenation reactions. Cyclopentadienones, for example, are ligands that might be useful for this type of reaction.
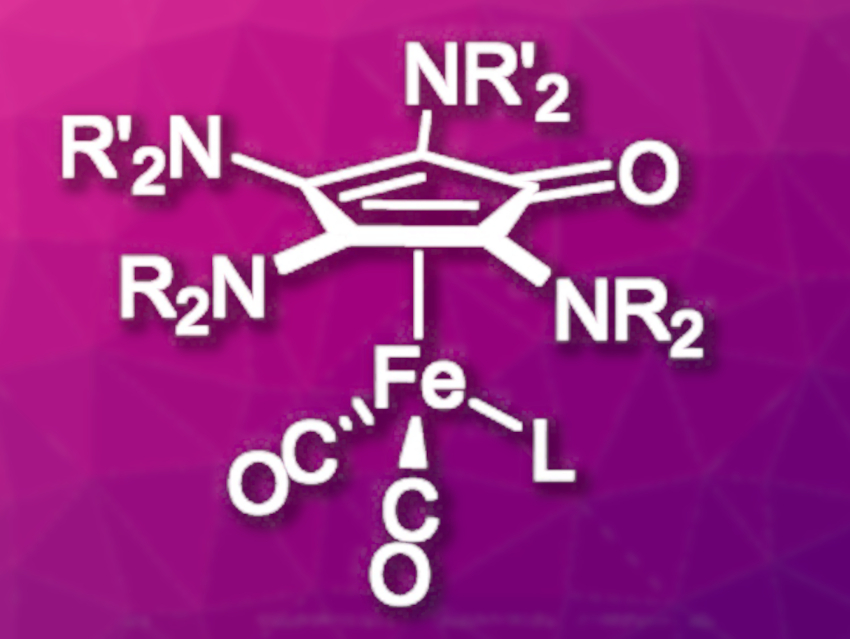
Matthias Tamm, Technical University of Braunschweig, Germany, and colleagues have developed a catalytic hydrogenation approach using tetraaminocyclopentadienone iron complexes (pictured, NR2/NR’2 = piperidinyl, 4-methylpiperidinyl, homopiperidinyl, L = CO, NC–CH3) as pre-catalysts. Syntheses starting from amino-substituted acetylenes were used for the preparation of the tetraaminocyclopentadienone complex. Symmetric ligands (R = R’) were synthesized directly by a one-step procedure from Fe(CO)5 and the respective diamino acetylene in tetrahydrofuran (THF) at room temperature. For the synthesis of asymmetric ligands, Fe(CO)5 was reacted with one equivalent of the first diamino acetylene at –78 °C to form a ferracyclobutenone intermediate. The addition of a second diamino acetylene to this intermediate at –60 °C then led to the desired complex.
To increase activity, one of the remaining three CO ligands was substituted by the more labile acetonitrile. The catalytic activity of the new complexes was tested in two different hydrogenation reactions: Transfer hydrogenations where the hydrogen atom is transferred from a protic solvent (in this case, isopropanol) to the substrate and pressure hydrogenations with elemental hydrogen at elevated pressure. The hydrogenation of ketones to the corresponding alcohols proceeded efficiently with up to 95 % yield. For more challenging, sterically demanding substrates, the transfer hydrogenation gave better results than the pressure hydrogenation. Imines were converted almost quantitatively under transfer hydrogenation conditions, while no conversion was observed under hydrogen pressure.
The new complexes have shown high activity in the tested reactions. The stepwise synthesis of asymmetric tetraaminocyclopentadienone ligands could offer the potential to develop asymmetric iron-catalyzed hydrogenations.
- Tetraaminocyclopentadienone Iron Complexes as Hydrogenation Catalysts,
Ludwig Hackl, Luong Phong Ho, Dustin Bockhardt, Thomas Bannenberg, Matthias Tamm,
Organometallics 2022.
https://doi.org/10.1021/acs.organomet.2c00075