Cationic polyene cyclizations are widely used in the total synthesis of terpenoid natural products. They can, for example, transform achiral polyolefins into substituted decalins (hydrocarbons with two fused six-membered rings). Asymmetric variants of this reaction usually give trans-fused decalins from (E)-polyene substrates.
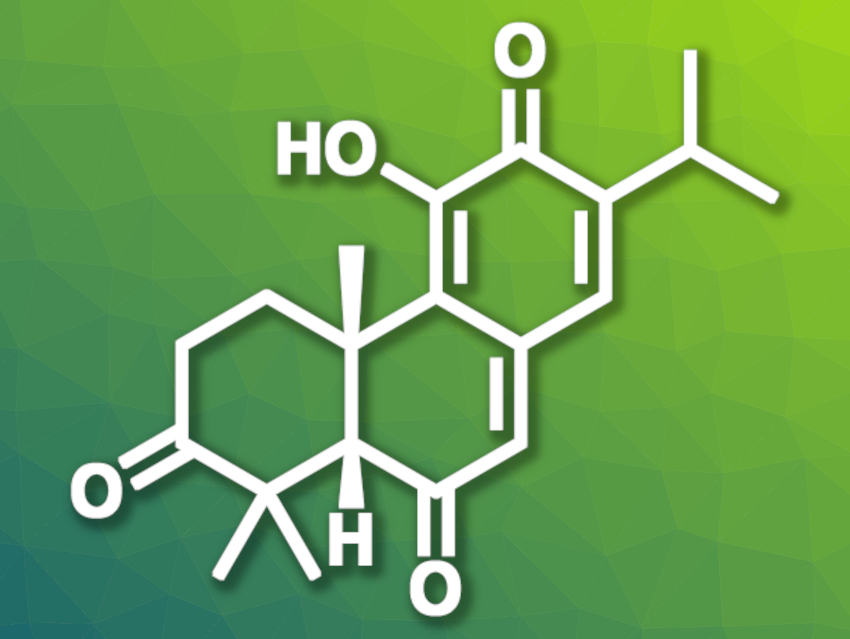
Samuel J. Plamondon and James L. Gleason, McGill University, Montreal, Canada, have found that chiral bicyclic hydrazide organocatalysts can be used to transform (Z)-polyenes into cis-decalin products. The team used this approach for the first total synthesis of the diterpenoid (−)-3-oxoisotaxodione (pictured above), which contains a cis-decalin unit.
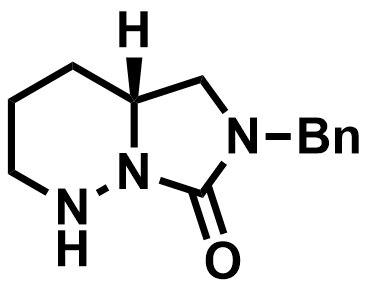
The researchers first prepared a suitable polyene precursor in five steps. The subsequent polyene cyclization to form the cis-decalin was performed on a gram scale in ethanol using a chiral bicyclic hydrazide catalyst (pictured below). The ketone group and the isopropyl substituent were then installed, followed by the two methyl groups at C4 and the quinone methide subunit.
The desired (−)-3-oxoisotaxodione was obtained in 19 steps overall. According to the researchers, the work demonstrates the utility of the employed bicyclic hydrazide as an organocatalyst for the cyclization of (Z)-polyene substrates to give cis-decalin frameworks with high enantioselectivity.
- Total Synthesis of (−)-3-Oxoisotaxodione,
Samuel J. Plamondon, James L. Gleason,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c00444