Biaryl compounds are useful, e.g., as ligands in asymmetric catalysis. They are often prepared via transition-metal-catalyzed cross-coupling reactions of prefunctionalized aryl building blocks. The oxidative coupling of two C–H bonds could be a useful alternative path to the formation of biaryl C–C bonds. However, controlling the site-selectivity in this type of reaction can be challenging. Biocatalytic oxidative cross-coupling reactions, in which the enzyme catalyst controls the selectivity, could allow researchers to overcome this limitation. Enzymes such as cytochromes P450 (P450s), for example, can promote the dimerization of phenolic compounds to give biaryl natural products.
Alison R. H. Narayan, University of Michigan, Ann Arbor, USA, and colleagues have investigated whether P450s can be used as biocatalysts for “unnatural” cross-coupling reactions to obtain biaryls. The team first used the cytochrome P450 enzyme KtnC, which they expressed using the yeast Pichia pastoris. They found that KtnC was able to promote the cross-coupling of different coumarins, leading to the formation of unnatural products. A diverse set of phenolic substrates could also be used as substrates. However, the reactivity and selectivity decreased as the substrate structures became less similar to the native coumarin framework.
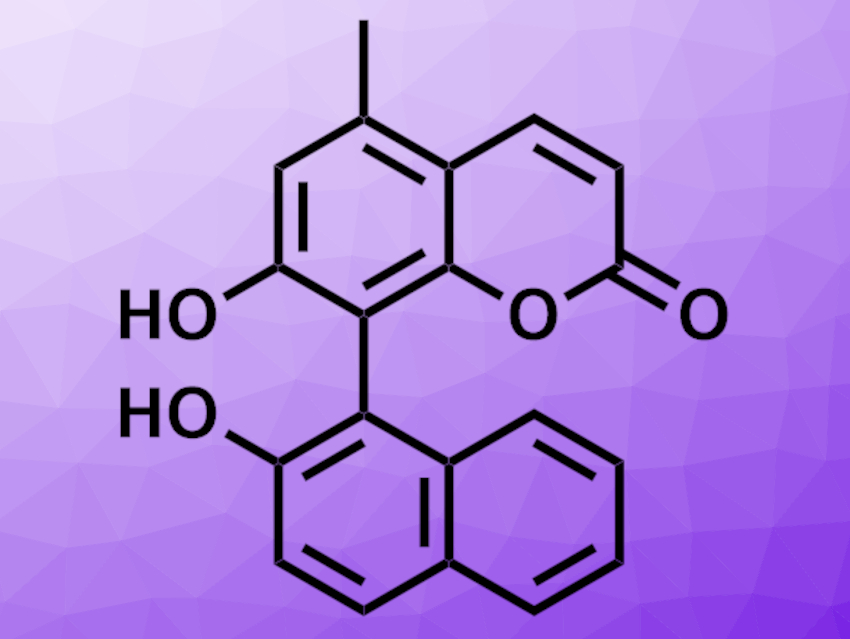
The researchers then engineered a P450 enzyme with the aim of increasing reactivity, site-selectivity, and stereoselectivity and making the biocatalytic cross-coupling practical for organic synthesis. They selected the cross-coupling product of a coumarin and 2-naphthol (pictured) as the target, which was obtained in low yields using the wild-type KtnC enzyme. The team then generated a large library of enzymes by introducing mutations and screened them for their catalytic properties in the target reaction. Through several rounds of directed evolution, the activity and selectivity in the desired reaction were then improved. The team obtained a biocatalyst with a 92-fold improvement in activity after five rounds of evolution. Further changes were made to increase the stereoselectivity, at the cost of a reduction in yield. According to the researchers, this biocatalytic approach could be a useful tool in the convergent assembly of biaryl compounds.
- Biocatalytic oxidative cross-coupling reactions for biaryl bond formation,
Lara E. Zetzsche, Jessica A. Yazarians, Suman Chakrabarty, Meagan E. Hinze, Lauren A. M. Murray, April L. Lukowski, Leo A. Joyce, Alison R. H. Narayan,
Nature 2022, 603, 79–85.
https://doi.org/10.1038/s41586-021-04365-7