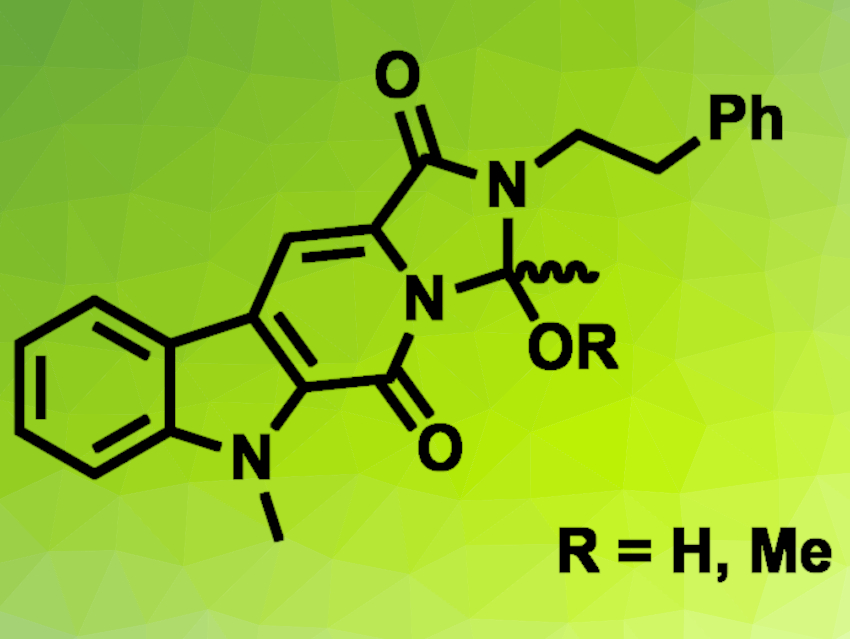
The spiro indole alkaloid cyanogramide has been isolated from the marine-derived bacterium Actinoalloteichus cyanogriseus. It has shown interesting bioactivities. The compound has a pyrrolo[1,2-c]imidazole system that is spiro-fused to an oxindole. In the biosynthesis of cyanogramide, cyanogramide B (pictured, R = H) is first converted to cyanogramide C (pictured, R = Me), and then further transformed to cyanogramide D, which has a styryl side chain, via a dehydrogenation reaction.
Thomas Lindel, Technische Universität Braunschweig, Germany, and colleagues have synthesized cyanogramides B and C, as well as their precursor marinacarboline E. The team first prepared marinacarboline E starting from L-tryptophan methyl ester using a Pictet–Spengler reaction, followed by methylation, saponification, and a coupling with 2-phenylethylamine.
Marinacarboline E was then subjected to a biomimetic oxidation to obtain cyanogramide B via an O-acetyl intermediate. A reaction of cyanogramide B with MeI/NaH in dimethylformamide (DMF) then gave cyanogramide C. According to the researchers, the work could contribute to the understanding of the enzymatic conversion of marinacarboline E to cyanogramide B.
- Biomimetic Synthesis of Cyanogramides B and C,
Dustin M. Sarnes, Peter G. Jones, Thomas Lindel,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c00510