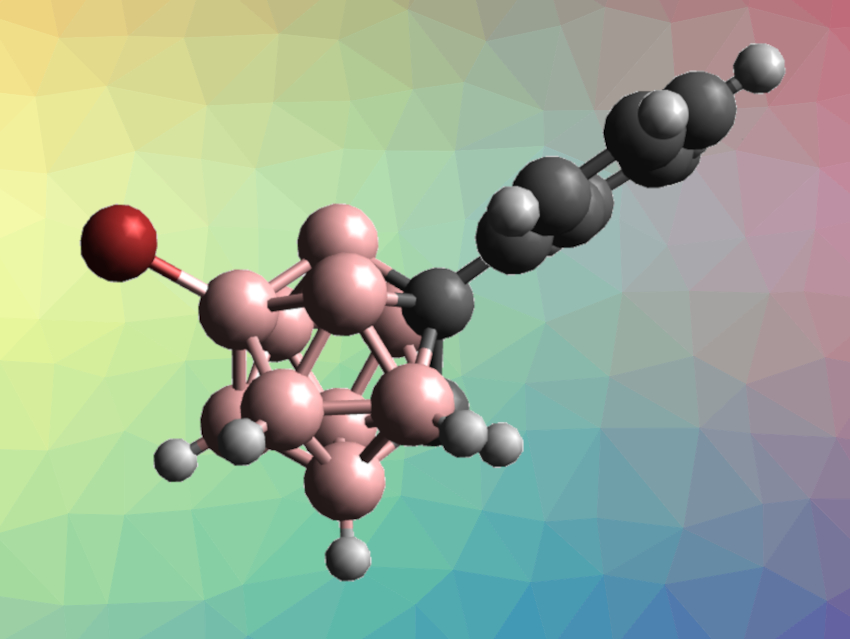
Carboranes are clusters composed of boron, carbon, and hydrogen atoms. They have applications, e.g., in optoelectronics, supramolecular chemistry, and coordination chemistry. The synthesis of functionalized carboranes is useful in this context. However, the selective functionalization of o-carboranes, for example, can be challenging. In particular, there is only a very small difference in electron density between the B(8)–H and B(9)–H vertices, which usually leads to a mixture of B(8)/B(9)-functionalized products.
Yan-Na Ma, Xuenian Chen, Zhengzhou University, China, and colleagues have developed a practical and environmentally friendly electrophilic B(9)–H halogenation of o-carboranes and m-carboranes with excellent selectivities (example product pictured) using N-haloamides in hexafluoroisopropanol (HFIP). The team used trichloroisocyanuric acid (TCCA), tribromoisocyanuric acid (TBCA), or N-iodosuccinimide (NIS) for chlorination, bromination, and iodination reactions, respectively.
The desired halogenated products were obtained in excellent yields when free o-carborane was used as the substrate. The team also halogenated a variety of substituted o-carboranes, as well as m-carborane. The process is metal- and catalyst-free as well as air- and moisture-tolerant, and the products can be isolated by a simple extraction with n-hexane.
- Practical Synthesis of B(9)-Halogenated Carboranes with N-Haloamides in Hexafluoroisopropanol,
Wenjing Guo, Chenyang Guo, Yan-Na Ma, Xuenian Chen,
Inorg. Chem. 2022.
https://doi.org/10.1021/acs.inorgchem.2c00074