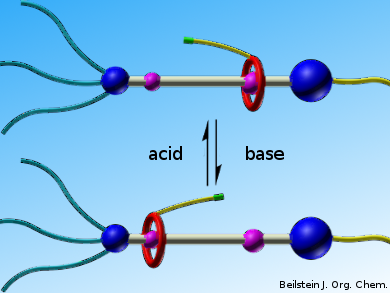
So far, the repertoire of available functional rotaxanes as building blocks for the preparation of stimuli-responsive polymers remains limited. The induction of active and functional groups make the preparation of rotaxanes more difficult and complicated. Therefore, it is of interest to enrich both the family of stimuli-responsive units and the methods for constructing this kind of smart materials.
Da-Hui Qu and colleagues, East China University of Science and Technology, Shanghai, have designed, synthesized, and characterized a functional [2]rotaxane with two alkenyl bonds on the macrocycle and the axle, respectively. A functional dibenzo-24-crown-8 (DB24C8) macrocycle is interlocked with a flexible chain. One side of the rotaxane chain is terminated by a bulky stopper bearing three long alkyl chains to prevent the macrocyclic moiety from slipping out the thread.
The macrocycle can reversibly shuttle between two different recognition sites (the dibenzylammonium (DBA) recognition site and the amide moiety) under the stimuli of external acid and base. Deprotonation of the DBA site by base makes the DB24C8 moiety slide to the amide station and the macrocycle could move back to the DBA recognition site when acid was added. The alkenyl bonds were chosen as the functional group due to their considerable stability during synthesis and their remarkable reaction activity. The naked alkenyl groups could react with other functional groups such as alkenyl and thiol groups to prepare stimuli-responsive polymers.
According to the researchers, this kind of rotaxane bearing functional groups is a powerful platform for preparing stimuli-responsive polymers.
- A switchable [2]rotaxane with two active alkenyl groups,
Xiu-Li Zheng, Rong-Rong Tao, Rui-Rui Gu, Wen-Zhi Wang, Da-Hui Qu,
Beilstein J. Org. Chem. 2018, 14, 2074–2081.
https://doi.org/10.3762/bjoc.14.181