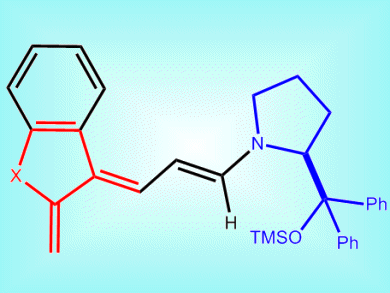
The Diels–Alder reaction is probably the most powerful technology for single-step constructions of complex chiral molecules. One highly useful class of dienes, ortho-quinodimethanes (oQDMs, pictured), has never been used for a catalytic enantioselective Diels—Alder reaction. This is due to the need to generate the reactive and unstable oQDMs dienes in situ and the harsh reaction conditions needed to do so.
Paolo Melchiorre and co-workers, ICIQ — Institute of Chemical Research of Catalonia, Tarragona, Spain, have achieved the first asymmetric catalytic Diels–Alder reaction of oQDMs under mild and simple reaction conditions. They generated the oQDMs in situ by using a chiral amine as a catalyst (blue section in picture). These then reacted with two classes of olefinic dienophiles, nitroolefins and methyleneindolinones.
This chemistry gives high regio-, diastereo-, and enantio-control. It can be extended to the synthesis of polycyclic pyrrole- or furan-based compounds and its simplicity should see it rapidly taken up in synthetic and medicinal chemistry.
- Asymmetric Catalysis of Diels–Alder Reactions with in Situ Generated Heterocyclic ortho-Quinodimethanes
Y. Liu, M. Nappi, E. Arceo, S. Vera, P. Melchiorre,
J. Am. Chem. Soc. 2011.
DOI: 10.1021/ja206517s
See also:
- Future Visions of Chemistry: Paolo Melchiorre
Interview with Paolo Melchiorre, ICIQ, Spain: “I am sure science will uncover and explain concepts that I cannot even imagine now.”