Solar energy conversion via water splitting to produce H2 fuel is an attractive alternative energy source. Complexes that both absorb solar light and photocatalytically split water are difficult to design as a delicate balance between steric and electronic effects must be struck.
Karen J. Brewer and colleagues, Virginia Tech, USA, have studied the known complex 1 and report the new Ru,Rh bimetallic complexes 2 and 3 (see figure). They find that complex 1 can photoreduce but does not catalyze H2O reduction, dimerizing upon Rh(I) formation instead. Increasing the steric bulk to tBu2bpy, complex 2, stopped dimerization but also increased electron density at Rh, preventing photocatalytic function.
The complex 3 achieved the correct balance of steric and electronic factors, undergoing photoinitiated electron collection and photocatalytically generating H2 from H2O, one of only a few motifs known to display both functions.
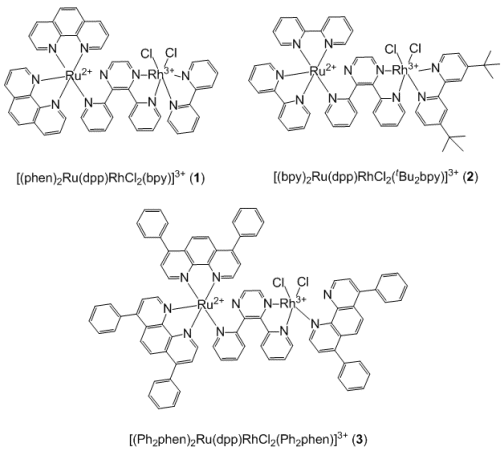
Figure 1. bpy = 2,2′-bipyridine, dpp = 2,9-diphenyl-1,10-phenanthroline, phen = phenanthroline.
- Discovering the Balance of Steric and Electronic Factors Needed To Provide a New Structural Motif for Photocatalytic Hydrogen Production from Water
T. A. White, B. N. Whitaker, K. J. Brewer,
J. Am. Chem. Soc. 2011.
DOI: 10.1021/ja206782k




