The report of the first N-heterocyclic carbene (NHC) by Arduengo and co-workers in 1991 triggered the tremendous development of such singlet carbenes from laboratory curiosities to powerful workhorses in synthesis and catalysis. They are most commonly generated by deprotonation of the corresponding azolium cations with a strong base. A limited number of NHCs have become commercially available, with the 1,2,4-triazol-5-ylidene derivative (1) being the earliest such example. A major drawback, however, is that prices exceed several hundred US$ per gram.
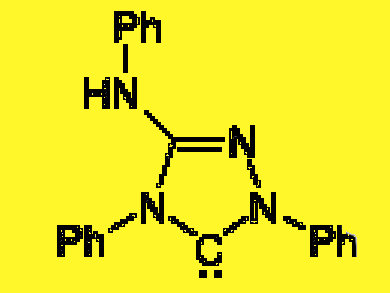
Ulrich Siemeling and colleagues, University of Kassel, Germany, show that the so far unrecognized reactivity of the well known analytical reagent Nitron (2) towards carbene trapping reagents reflects the presence of a NHC-type tautomer in solution (2′). This tautomer, which is closely related to Enders’ 1,2,4-triazol-5-ylidene (1), has never been considered before. This instant carbene has been commercially available for more than a century, and is by far the cheapest NHC.
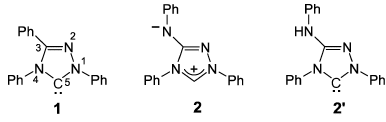
- Nitrogen: a stable N-heterocyclic carbene that has been commercially available for more than a centura
Christian Färber, Michael Leibold, Clemens Bruhn, Martin Maurer, Ulrich Siemeling,
Chem. Commun. 2011.
DOI: 10.1039/c1cc16460k