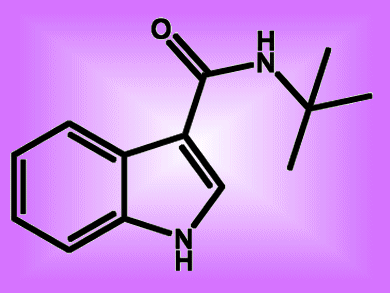
Researchers at the Chinese Academy of Sciences, Guangzhou, have developed a direct transition-metal-catalyzed approach to functionalizing indoles — useful building blocks in natural product chemistry and the synthesis of pharmaceutical products.
Carboxamidation with isocyanide insertion is possible at the C3 carbon with Pd(OAc)2 as catalyst and Cu(OAc)2 as the stoichiometric oxidant, the team explains. The researchers point out that during the last hundred years or more, chemists have developed several complementary approaches to functionalized indoles, including regioselective arylation, alkenylation, alkylation, cyanation, carbonylation and borylation. They are, the team believes, the first to use this approach for making indole-2(3)-carboxamides, which have remarkable bioactivities and could be used as building blocks for more complicated derivatives.
- Direct carboxamidation of indoles by palladium-catalyzed C−H activation and isocyanide insertion,
J. Peng, L. Liu, Z. Hu, J. Huang, Q. Zhu,
Chem. Commun. 2012.
DOI: 10.1039/C2CC30351E