For many decades industry has been producing methanol on a large scale from a mixture of carbon dioxide and carbon monoxide, as well as hydrogen. Researchers so far only studied the catalytic process on idealized model systems. An international team around Malte Behrens, Fritz Haber Institute of the Max Planck Society, Berlin, Germany, now clarified why the catalyst used works so well.
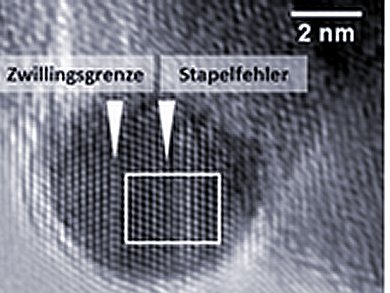
The industrial catalyst is composed of innumerable nanoparticles, some made of copper, some of zinc oxide and a small proportion of aluminium oxide. Together they form a type of nanosponge. Using images from a high-resolution transmission electron microscope (HRTEM) and neutron diffraction, which provides information on the crystal structure, the scientists discovered defects in the arrangement of the copper atoms in the nanoparticles. Quantum chemical computations prove that some of the intermediate products prefer to adsorb at these defects, showing that the defects increase the catalyst’s activity.
Synchrotron radiation experiments and HRTEM images show that zinc oxide creeps over parts of the copper particles, and that some atoms in the copper surface are replaced by zinc. This also makes the catalyst very active: calculations showed that some intermediate products of the reaction – in this case those containing oxygen – are more likely to bond to the zinc than to the copper.
These findings could make a contribution to further improving the catalyst, and also help researchers develop catalysts that convert pure carbon dioxide efficiently.
Image: © Malte Behrens/Fritz Haber Institute of the MPG
- Max Planck Society, Berlin, Germany