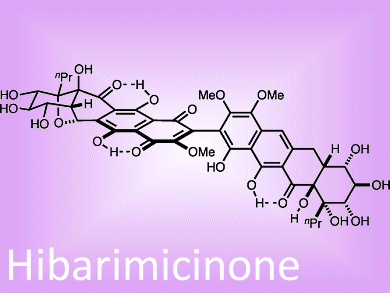
Matthew Shair and colleagues, Harvard University, USA, have developed a novel strategy that allowed them to construct, and then characterize for the first time, the hibarimicins, including hibarimicinone (pictured). These pseudodimeric type-II polyketides were originally isolated from a culture broth of the rare actinomycete Microbispora rosea and have been shown to block the proliferation of a wide range of cancer cell types in the laboratory.
The team exploited a newly devised benzyl fluoride Michael–Claisen reaction sequence to build the complete carbon skeleton of two of these molecules, HMP-Y1 and atrop-HMP-Y1. Symmetrical, two-directional, double annulations allowed them to pull together the two halves of the molecule, each of which bears four fused carbon rings. The total synthesis required only a further three to five steps after this double annulation to make the final product and a related compound.
- Total Syntheses of HMP-Y1, Hibarimicinone, and HMP-P1,
Brian B. Liau, Benjamin C. Milgram, Matthew D. Shair,
J. Am. Chem. Soc. 2012.
DOI: 10.1021/ja307207q