Biodiesel fuel is an alternative for fossil fuel and is produced by a transesterfication reaction of triacylglycerols with methanol. The most popular reaction uses NaOH or KOH as catalyst and an excess of methanol to ensure a high conversion. Once the reaction is finished, the biodiesel fuel has to be purified to avoid subsequent operational problems in engines caused by contaminants. Therefore, biodiesel and free glycerol are first allowed to separate into two layers. The crude biodiesel obtained from the upper layer still contains some impurities including residual methanol, alkali metals from the catalyst, as well as other contaminants.
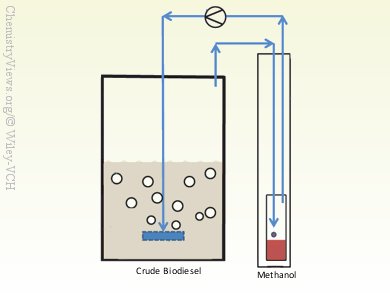
Masao Yukumoto and colleagues, Chubu Univerisity, Kasugai City, Japan, have developed a simple, compact, and efficient system to remove the residual methanol from the crude biodiesel by bubble stripping. Gas is supplied by a gas pump through a gas bubbler to the crude biodiesel. The exit gas containing methanol vapor is then passed through a condenser and the liquid methanol is collected in a vessel. The gas can then be returned to the pump and is thereby circulated in a closed system. Sediment that appears after bubble stripping is subsequently removed by centrifugation. In the final step, trace amounts of potassium and free glycerol are removed by absorbent treatment.
As a new method to remove methanol from crude biodiesel, bubble stripping is a simpler and more efficient refinery process than the previous processes used and will be applicable to an industrial production of high-quality biodiesel.
- Bubble stripping in closed system to remove residual methanol from crude biodiesel,
Tsuneo Yamane, Ryosuke Achiha, Tomoaki Namioka, Masao Yukumoto,
Eur. J. Lipid Sci. Technol. 2013.
DOI: 10.1002/ejlt.201300134