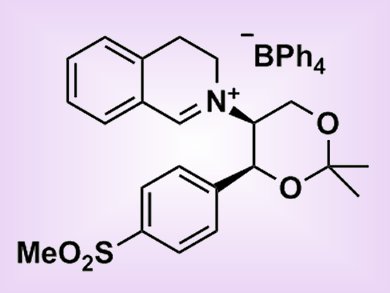
The formation of chiral epoxides through asymmetric epoxidation reactions of alkenes is synthetically useful and a number of methods to generate these compounds exist. One method involves the use of chiral catalysts containing an iminium salt; with the chiral fragment attached to the nitrogen atom of the imine.
Through their work to synthesize biologically-active natural products, Philip Bulman Page and colleagues, University of East Anglia, Loughborough University, University of St. Andrews, Nottingham Trent University, and Charnwood Molecular Ltd, UK, noted that epoxidation of alkenes with these iminium-salt catalysts (see picture) resulted in very high enantioselectivities. Considering these results, the salts were evaluated as candidates for kinetic resolution — a way to preferentially form one enantiomer over another owing to differing rates of formation. One catalyst, which performed best with chromenes, was tested on a range of compounds and acceptable yields (typically 40–50%) and good enantioselectivities (typically 80% ee) were achieved.
The best results were obtained with substrates containing nitro and cyano groups. This new method could be used in an array of synthetic approaches.
- Kinetic Resolution in Asymmetric Epoxidation using Iminium Salt Catalysis,
Philip C. Bulman Page, Louise F. Appleby, Yohan Chan, David P. Day, Benjamin R. Buckley, Alexandra M. Z. Slawin, Steven M. Allin, Michael J. McKenzie,
J. Org. Chem. 2013.
DOI: 10.1021/jo401345m