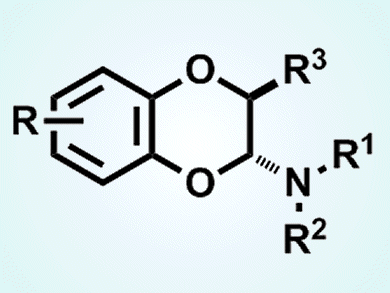
The 1,4-benzodioxin skeleton is a common starting point for organic synthesis in natural product and pharmaceutical chemistry. Until now biomimetic approaches that have several drawbacks have been the main route to this structure. However, they are not the only way to build this starting material according to Jinsong Zhang, California State University, Chico, CA, USA, and colleagues there and at the University of California, Davis, CA, USA.
They have used an inverse electron demand hetero-Diels–Alder reaction to prepare the compound from o-quinone and enamine. The team used a hypervalent iodine reagent to carry out the reaction, which proceeds under mild reaction conditions. Proof of principle was demonstrated with the synthesis of a range of derivatives, one of which was found to have antimicrobial activity.
The next step will be to develop a regioselective version of the reaction and so preclude the need to separate isomers in the final stages.
- Inverse electron demand hetero-Diels–Alder reaction in preparing 1,4-benzodioxin from o-quinone and enamine,
Jinsong Zhang, Chris Taylor, Erich Bowman, Leo Savage-Low, Michael W. Lodewyk, Larry Hanne, Guang Wu,
Tetrahedron Lett. 2013, 54, 6298–6302.
DOI: 10.1016/j.tetlet.2013.09.013