The mantis shrimp, which evolved more than 300 million years ago, has 16 wavelength-specific opsins in its compound eye: more than in any other animal known. It is a master of tuning the common chromophore, 11-cis-retinal, via modifications to its protein “receptacle”, a membrane protein known as an “opsin”, a group of light-sensitive membrane-bound protein-coupled receptors, the most ancient of which – bacteriorhodopsin – pump protons in response to light.
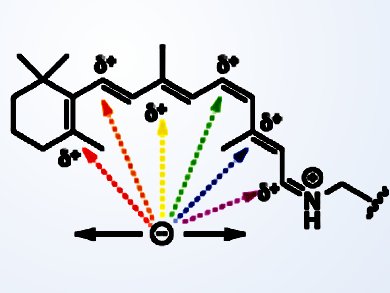
Wenjing Wang, Massachusetts Institute of Technology (MIT), Cambridge, MA, USA and colleagues from Michigan State University, East Lansing, MI, USA, have demonstrated wavelength tuning over the entire visible range from 425 to 644 nm by modifying the protein opsin in nine positions via transgenic expression in bacteria. There was a major challenge in tuning retinal’s absorption spectrum in its protein “receptacle”: because the ionone ring of retinal is exposed to the solvent, solvent-dependent ionic interactions overwhelm the influence of the binding pocket in the protein, which is largely hydrophobic. Mutations made in the opsin’s retinal binding pocket, therefore, have very little effect on the absorption spectrum. However, make the aliphatic chain of the retinal shorter, and the ionone ring moves in further into the binding pocket, and is no longer so buffered by the solvent. Upon mutation of the opsin, retinal’s absorption maximum can now change significantly.
The search for proteins that bind retinal even more deeply – hence de-buffering the ionone ring even more – led to human cellular retinol binding protein II (hCRBPII), which accommodates retinal completely within the binding pocket. Now retinal’s spectroscopic properties could be studied and manipulated without solvent buffering. Using this system, and variants of it, a whole range of spectroscopically “tuned” rhodopsim mimics, with a variety of chromophores, has been produced. Applications in biotechnology beckon: for example red shifted microbial rhodopsin engineering could lead to near infra red-sensitive phototaxis and ion channels that open in response to heat.
- The photochemical determinants of color vision, Revealing how opsins tune their chromophore’s absorption wavelength,
Wenjing Wang, James H Geiger, Babak Borhan,
BioEssays 2013.
DOI: 10.1002/bies.201300094