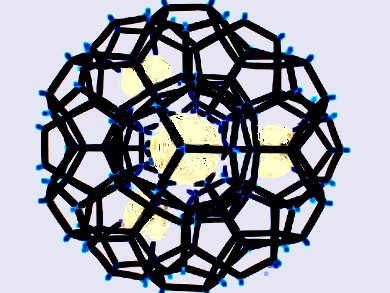
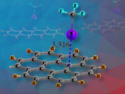
Clathrates are chemical compounds that have lattice-trapped guest molecules. For example, water can provide clathrate lattices to trap gaseous molecules, such as methane, due to its hydrogen-bonding networks. Inert as they are, even noble gases may form stable hydrate clathrates.
Neon hydrate, the last member of noble gas clathrates, has just been confirmed by a multi-international team led by Yusheng Zhao, University of Nevada, Las Vegas, Nevada, USA, using in situ neutron diffraction. At 480 MPa and 260 K, neon atoms are trapped in the water channels of ice II lattice mainly through van der Waals interactions, and their movements are only slightly restricted because of the lack of direct bonding.
This study has revealed a rare example of self-stabilized hydrate clathrates. In addition to answering the long-standing question whether neon can form hydrate, the results considerably contributes to the understanding of clathrates that bears potentials for energy storage and CO2 sequestration.
- Crystal structure and encapsulation dynamics of ice II-structured neon hydrate,
X. Yu, J. Zhu, S. Du, H. Xu, S. C. Vogel, J. Han, T. C. Germann, J. Zhang, C. Jin, J. S. Francisco, Y. Zhao,
Proc. Natl. Acad. Sci. U.S.A. 2014, 111, 10456–10461.
DOI: 10.1073/pnas.1410690111