Welwitindolinones belong to the family of terpenoid indole alkaloids and are isolated from cyanobacteria. They show fascinating structural complexities and diverse biological activities, but their biochemical origins are virtually unknown.
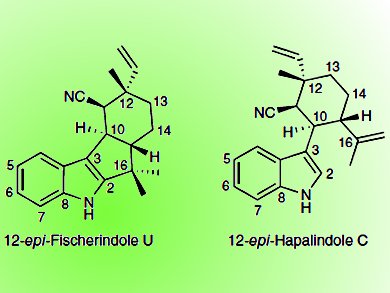
Xinyu Liu and Matthew L. Hillwig, University of Pittsburgh, PA, USA, recently identified and characterized the biosynthetic gene cluster of a welwitindolinone (wel) from the cyanobacterium Hapalosiphon welwitschii [1] and now were able to identify a nonheme iron enzyme (WelO5) in the wel biosynthetic pathway that can monochlorinate an aliphatic carbon in 12-epi-fischerindole U and 12-epi-hapalindole C. Enzyme-mediated reactions that regio- and stereospecifically add a halogen atom to an unactivated sp3 carbon in a freestanding molecule were so far unknown.
WelO5 is a Fe(II)/α-KG–dependent halogenase. It stereospecifically replaces a pro-R hydrogen at the C13 of the hapalindole-type molecules 12-epi-fischerindole U and 12-epi-hapalindole C with a chlorine. As WelO5 is pathway-specific, the team proposes that other organisms that produce hapalindole-type alkaloids encode analogous halogenases that are responsible for this dedicated chlorination reaction.
According to the team this finding together with further structural and biochemical studies of WelO5 and related halogenases, will lead to the development of catalysts for selective late-stage halogenations on unactivated carbons in complex moleculs.
- A new family of iron-dependent halogenases acts on freestanding substrates,
Matthew L Hillwig, Xinyu Liu,
Nature Chem. Biol. 2014.
DOI: 10.1038/nchembio.1625
[1] M. L. Hillwig, H. A. Fuhrman, K. Ittiamornkul, T. J. Sevco, D. H. Kwak, X. Liu, Identification and Characterization of a Welwitindolinone Alkaloid Biosynthetic Gene Cluster in Stigonematalean Cyanobacterium Hapalosiphon welwitschii, ChemBioChem 2014, 15, 665–669. DOI: 10.1002/cbic.201300794
- On the Origin of Alkaloids,
ChemViews Mag. 2014.