C–H activation reactions are a useful and powerful tool in many synthetic reactions. Although many protocols exist, control of the regioselectivity is often problematic. Recently, a handful of approaches have tried to overcome this drawback.
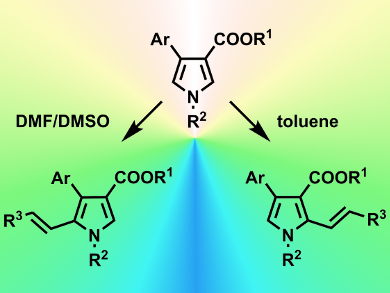
Aijun Lin, Hequan Yao, and colleagues, China Pharmaceutical University, Nanjing, have developed a switchable C–H alkenylation reaction that selects between two possible regiochemical outcomes simply by altering the solvent the reaction is performed in. The reaction chosen was the C–H alkenylation of a 3,4-disubstituted pyrrole derivative under Heck conditions (Pd(OAc)2 catalyzed). A range of solvents was screened against a number of pyrrole and double-bond reagents. Both regiochemical products (addition either at the C2 or C5 position) were obtained in good yields (≈80 %) and selectivities (up to >19:1 for C2 in toluene and 13:1 for C5 in DMSO/DMF).
To highlight this method’s potential, the C5-selective reaction was applied to the key Heck reaction required to prepare (±)-rhazinilam (a natural product) and resulted in 56 % yield and 12:1 selectivity.
- Solvent-Controlled Switchable C–H Alkenylation of 4-Aryl-1H-pyrrole-3-carboxylates: Application to the Total Synthesis of (±)-Rhazinilam,
Youla Su, Haipin Zhou, Jiaxuan Chen, Jinyi Xu, Xiaoming Wu, Aijun Lin, Hequan Yao,
Org. Lett. 2014.
DOI: 10.1021/ol5023933