A major contributor to global warming and a cause of acid rain, carbon dioxide, is on the other hand a promising C1 building block for fuels and chemicals through reductive transformations.
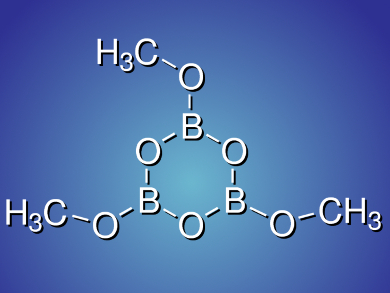
During their study using transition metals to reduce CO2 with BH3, Tsutomu Mizuta and colleagues, Hiroshima University, Higashi-Hiroshima, Japan, discovered a more straightforward route to serve that purpose. When they treat commercially available BH3•THF solution stabilized with 0.5 mol% NaBH4 with 1 atm CO2 at room temperature, they obtain trimethoxyboroxine in 87 % yield. The reaction is presumably catalyzed by the BH4– anion.
As a new preparation method for trimethoxyboroxine, a common electrolyte additive, this process only requires inexpensive commercial reagents, and does not necessitate any catalyst, making it both convenient and atomically economic.
- Reduction of CO2 to Trimethoxyboroxine with BH3 in THF,
Koji Fujiwara, Shogo Yasuda, Tsutomu Mizuta,
Organometallics 2014.
DOI: 10.1021/om5008488