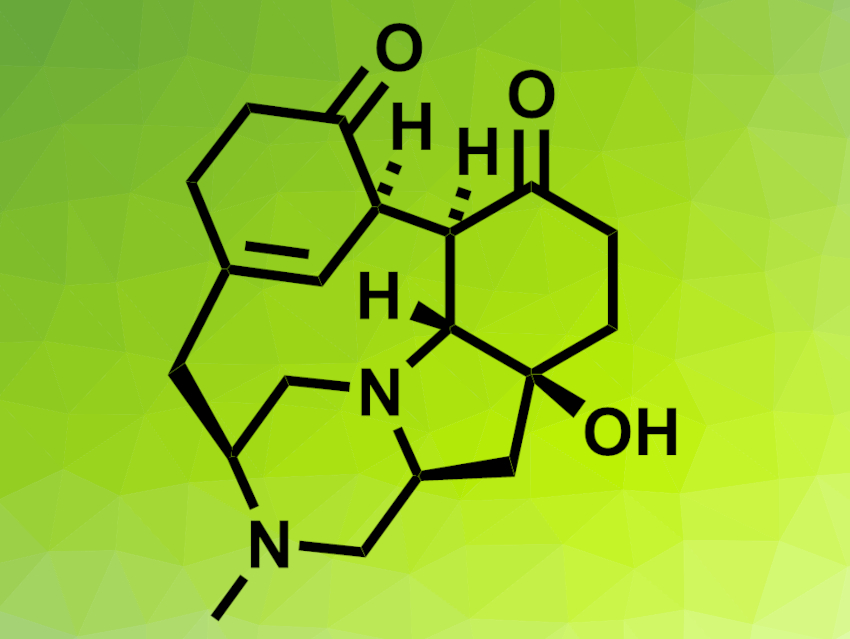
Herquline A was first isolated from a fungus and has a highly strained bowl-shaped pentacyclic structure. It has shown interesting bioactivities. However, the compound is a very challenging synthetic target and there has been no total synthesis of herquline A so far.
Sunkyu Han, Korea Advanced Institute of Science and Technology (KAIST) and Institute for Basic Science (IBS), Daejeon, Republic of Korea, and colleagues have synthesized 1-hydroxyherquline A (pictured) and attempted to convert it to herquline A. The team started from a tetracyclic ketone precursor, which was reduced to an alcohol. After the introduction of an epoxide group and a Birch reduction, the researchers introduced a protecting group at a free secondary amine and re-oxidized the alcohol. The installation of a hydroxyl group at C1, followed by an aza-Michael addition-mediated N–C bond formation to close the final ring, then gave 1-hydroxyherquline A.
The team attempted to reduce 1-hydroxyherquline A to obtain herquline A. However, the tertiary alcohol moiety did not undergo the desired reaction. The researchers propose that this is due to the steric congestion around the site, which is positioned inside the “bowl-shaped” cavity of the substrate. According to the team, the work could serve as a stepping stone for other synthetic approaches towards herquline A.
- Synthesis and Reactivity of 1-Hydroxyherquline A,
Thomas T. Kim, Chungwoo Lee, Dongwook Kim, Hee-Seung Lee, Sunkyu Han,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c00379