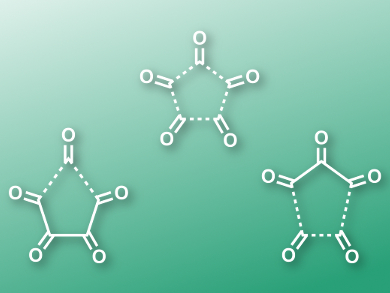
Monocyclic oxocarbons (CO)n are interesting molecules known since the 1960s. While extended chains of carbon monoxide units are unknown, small rings are fairly stable. Nevertheless, the dissociation of uncharged species into separate CO molecules is highly exothermic.
Xiaoguang Bao, Soochow University, Suzhou, China, Weston Thatcher Borden, University of North Texas, Denton, USA, and colleagues have calculated the likely mechanism of the fragmentation of 1,2,3,4,5-cyclopentanepentone into five molecules of CO using both density functional theory (DFT) and ab initio methods. The team found that while the reaction is allowed by the Woodward-Hoffmann rules, the molecule does not fragment into five CO units in one step via a symmetric transition state (pictured in the center).
There exist two possible pathways with lower activation energies, in which either one or two CO molecules are lost in the first step (pictured left and right, respectively). The latter leads to C3O3, which then dissociates without a barrier into three molecules of CO. The researchers explain the lower energy of these transition states compared to the symmetric pathway with second-order Jahn−Teller (SOJT) distortions.
- Theoretical Analysis of the Fragmentation of (CO)5: A Symmetry-Allowed Highly Exothermic Reaction that Follows a Stepwise Pathway,
Jiajun Liu, Xiaoguang Bao, David A. Hrovat, Weston Thatcher Borden,
J. Org. Chem. 2015.
DOI: 10.1021/acs.joc.5b01546