Hypoxia is a state of oxygen deprivation characteristic of many solid cancerous tumors. Oxygen starvation helps tumors resist both radiotherapy and chemotherapy. Treatment with small interfering RNA (siRNA) is toxic to such tumors through a process known as gene silencing, but getting these nucleic acids deep into the tumor is challenging.
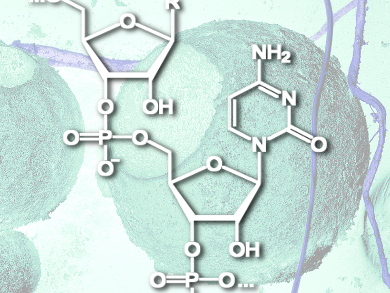
Hypoxic cancer cells consume more glucose than healthy cells, an effect that Wei Zhang, Nantong University, China, and colleagues hope to use in order to guide siRNA to its target. They covered quantum dots with a polyarginine peptide (for nucleic acid binding), covalently linked to a glucose-like molecule (for tumor targeting). The linker was a pH-sensitive hydrazone, designed to break after cell uptake, so that the nanoparticle delivers its bound siRNA instead of being recycled by the cell’s endosomes.
The team tested this nanoparticle construct for its ability to bind siRNA and deliver it into cancer cells in a range of in vitro and in vivo experiments. They observed gene silencing, anticancer activity, and less organ toxicity than quantum dots alone in mice. Furthermore, as quantum dots are fluorescent, the nanoparticles could be tracked in real time around the body of the mouse, revealing excellent in vivo tumor targeting.
- pH-responsive hybrid quantum dots for targeting hypoxic tumor siRNA delivery,
HongYan Zhu, ShengYu Zhang, Yong Lin, GuoLiang Meng, Yu Yang, Wei Zhang,
J. Control. Release 2015.
DOI: 10.1016/j.jconrel.2015.11.017



It is known from experimental studies that metformin, a potent anti-hyperglycemic agent, induces apoptosis. Cytosolic NADPH might be limited for cell proliferation because its level is critical for providing reducing equivalents for fatty acid and cholesterol biosynthesis, as well as for modulating oxidative stress. tosis of CLL cells. The main effect of this drug from the biguanide family is to acutely decrease hepatic glucose production, mostly through a mild and transient inhibition of the mitochondrial respiratory-chain complex. In addition, the result decreases liver energy and activates the AMP-activated protein kinase (AMPK), a cellular metabolic sensor. Activated AMPK switches cells from an anabolic to a catabolic state, shutting down the ATP-consuming synthetic pathways and restoring energy balance.
It has been suggested that metformin could inhibit the growth of cancer cells by decreasing cellular energy status, and it could force a metabolic conversion that cancer cells are unable to execute. A recent study revealed that AMPK activation promotes the survival of cells metabolically impaired by glucose limitation in part through p53 activation. Also, the tumor-specific mutations in IDH1 and IDH2 have resulted in a loss of their normal enzymatic activity of interconvert isocitrate at ketoglutarate acid expressed in hematopoietic malignant cells. In earlier studies, it was highlighted that epigenetic abnormalities may have the potential to increase the risk of tumor-genesis. Mechanisms of chromatin remodeling include dynamic interplay among ATP-dependent complexes, covalent histone modifications, utilization of histone variants and DNA methylation. Chromosomal rearrangements studies in chronic lymphocytic leukemia led to the hypothesis that cancer cells may undergo catastrophic events, whereas in the genome, a large number of rearrangements are required within a single breakage–fusion events.