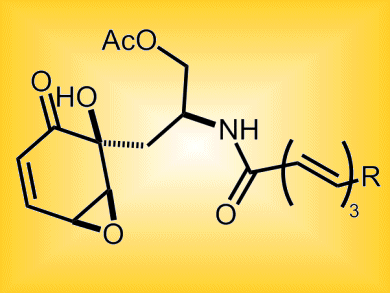
Scyphostatin is a potent reversible inhibitor of neutral sphingomyelinases (N-SMases), which are implicated in ceramide production and subsequent cell apoptosis (cell death). Its hydrophobic side chain moiety is thought to play a critical role in controlling both the reversibility and selectivity of N-SMase inhibition.
Thomas Pettus and co-workers, University of California, Santa Barbara, USA, have developed a strategy for the synthesis of scyphostatin analogues which permits late-stage manipulation of the hydrophobic chain while maintaining the epoxycyclohexenone motif.
A carboxylic acid cyclization component was used due to the incompatibility of sulfonamide-based cyclohexadienones with the epoxidation. The generation of enantiomerically pure cyclohexadienones was elucidated and could be employed in an epoxidation protocol without compromising the stereochemical integrity of the core.
- A Strategy for the Late-Stage Divergent Syntheses of Scyphostatin Analogues
J. Y. Cha, G. L. Burnett, Y. Huang, J. B. Davidson, T. R. R. Pettus,
J. Org. Chem. 2011.
DOI: 10.1021/jo102327e




It is a good work to some degree! Prof. Dr. Amgad M. Rabie …