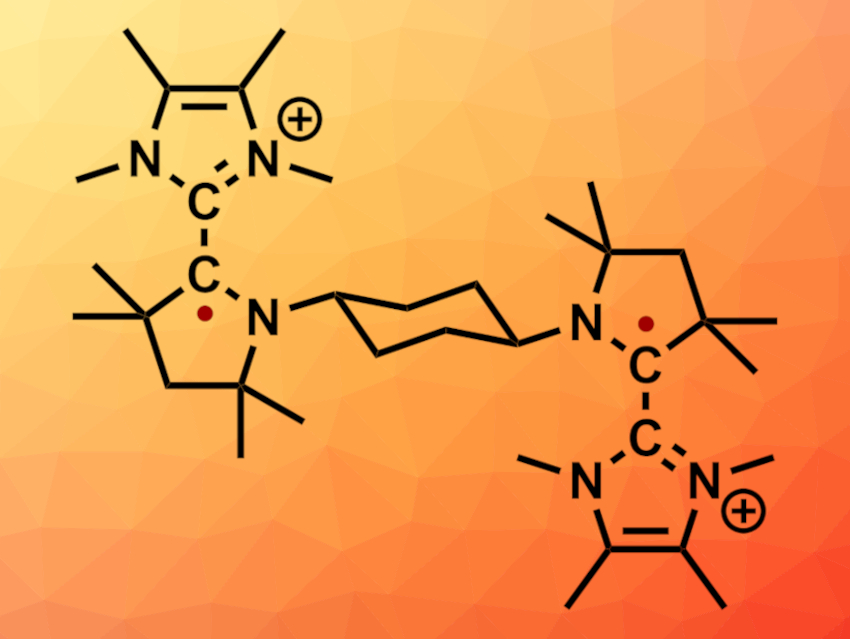
Diradical compounds are interesting research targets, e.g., in organic and physical chemistry. In this context, carbon-centered diradicals are less well studied than heteroatom-centered diradicals. This is due to their generally lower stability. One promising approach for the synthesis of carbon-centered diradicals involves the use of redox-active organic compounds that are subjected to electron-transfer reactions.
Abhishake Mondal, Indian Institute of Science, Bangalore, India, Ramamoorthy Boomishankar, Indian Institute of Science Education and Research Pune, India, Swapan K. Pati, Jawaharlal Nehru Centre for Advanced Scientific Research, Bangalore, India, Holger Braunschweig, Prince Ravat, University of Würzburg, Germany, Carola Schulzke, University of Greifswald, Germany, Anukul Jana, Tata Institute of Fundamental Research Hyderabad, India, and colleagues have synthesized a thermally stable dicationic diradical (pictured) based on dimers of N-heterocyclic carbenes (NHCs) and cyclic(alkyl)(amino)carbenes (CAACs), bridged by a trans-1,4-cyclohexylene unit.
The team started from a cyclohexylene-bridged bis-CAAC precursor, which was reacted with two equivalents of the NHC to obtain a dicationic intermediate. This intermediate was reacted with lithium diisopropylamide (LDA) to obtain the neutral bis-NHC-CAAC dimer. A four-electron oxidation with AgOTf then gave a tetracationic derivative, which reacted 1:1 with the neutral bis-NHC-CAAC dimer to give the desired dicationic diradical in the form of its OTf– salt as a red crystalline solid.
The structure of the product was confirmed using X-ray diffraction. The team also investigated the influence of the bridging unit between the two NHC-CAAC dimers. They synthesized ethylene- or propylene-bridged bis-NHC-CAAC dimers and found that they can undergo four-electron oxidation with AgOTf, but do not form stable dicationic diradicals. Instead, hydrogen abstraction from the bridging unit leads to the formation of a double bond. This reaction is hindered in the trans-1,4-cyclohexylene bridge. Thus, the cyclohexylene bridge is important for the successful isolation of the dicationic diradical.
- A bis-NHC-CAAC dimer derived dicationic diradical,
Mithilesh Kumar Nayak, Pallavi Sarkar, Benedict Josua Elvers, Sakshi Mehta, Fangyuan Mrs. Zhang, Nicolas Dr. Chrysochos, Ivo Krummenacher, T. Vijayakanth, Ramakirushnan Surya Narayanan, Ramapada Dolai, Biswarup Mr. Roy, Vishal Mr. Malik, Hemant Rawat, Abhishake Mondal, Ramamoorthy Boomishankar, Swapan K. Pati, Holger Braunschweig, Carola Schulzke, Prince Ravat, Anukul Jana,
Chem. Sci. 2022.
https://doi.org/10.1039/D2SC03937K